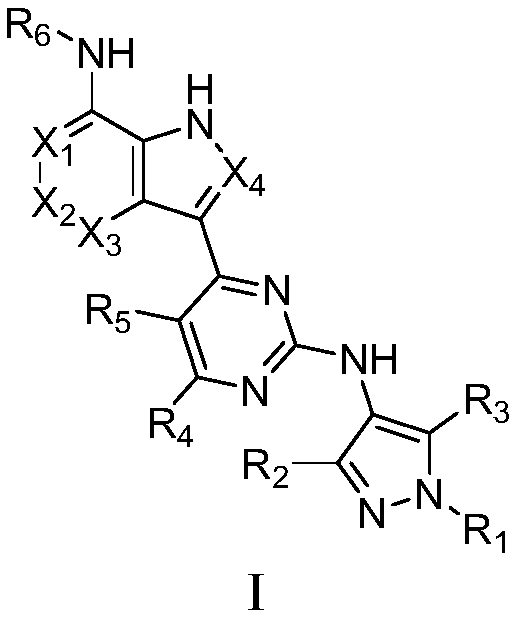
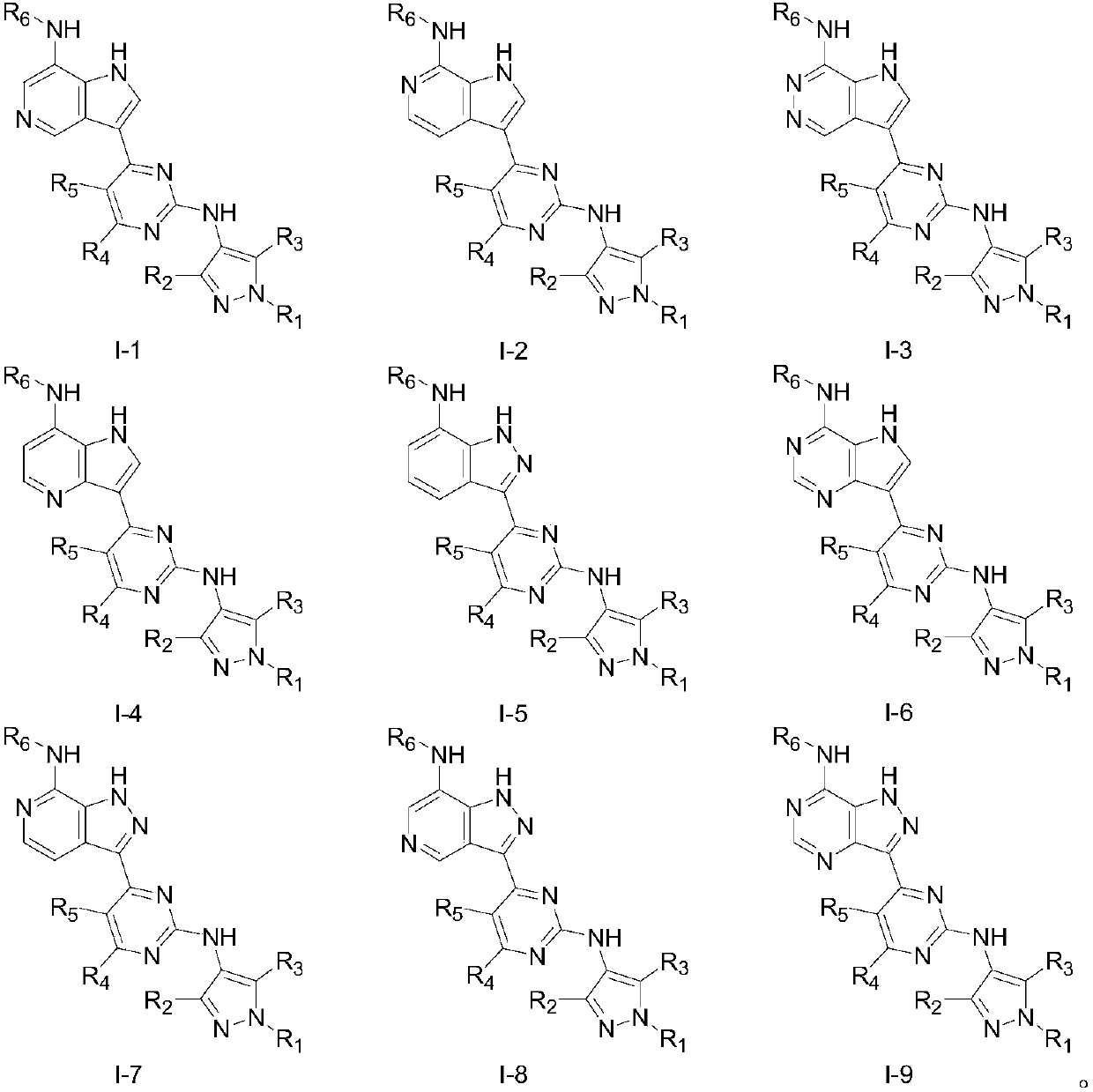
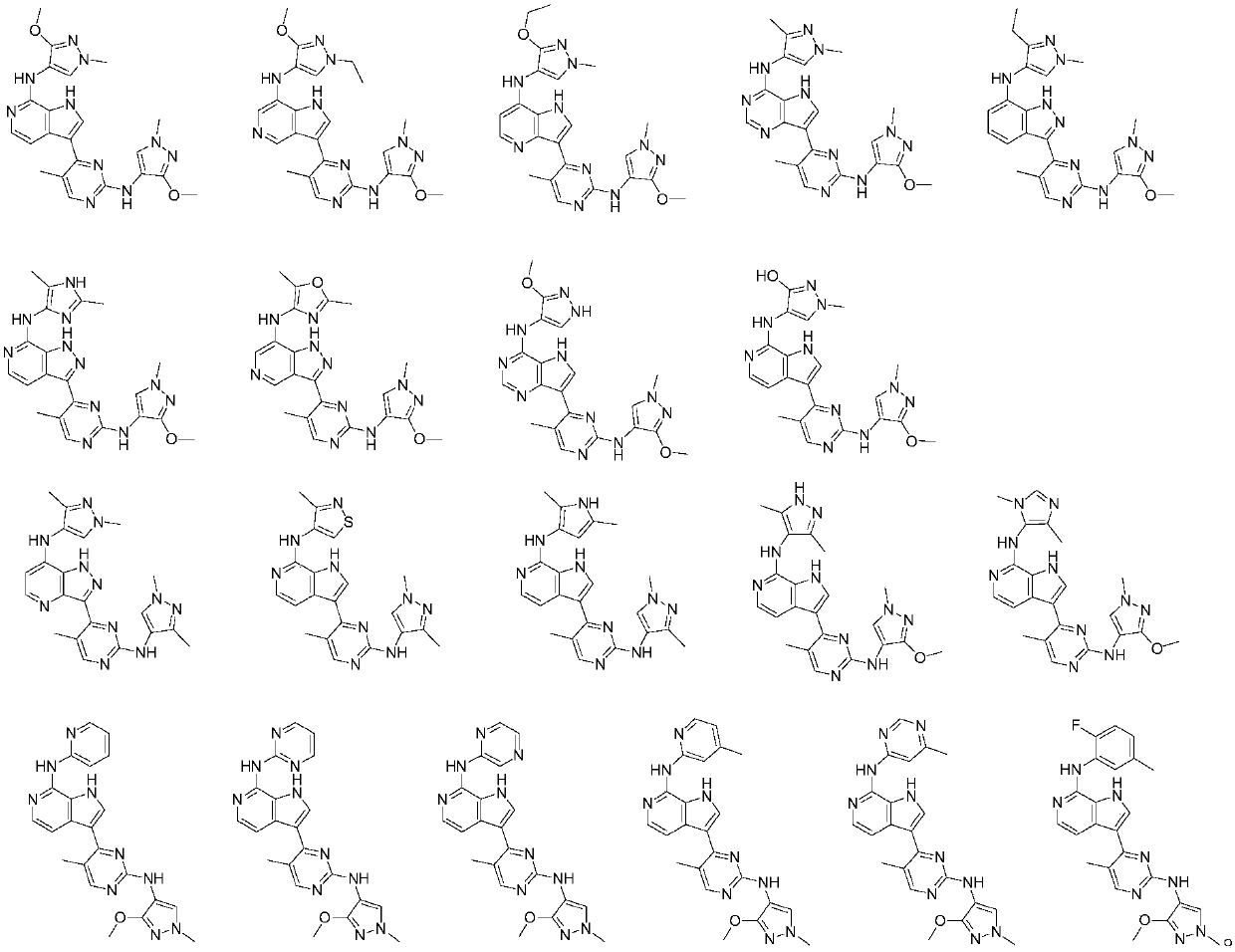
A class of aromatic heterocyclic compounds with kinase inhibition activity
A compound and aryl technology, applied in the field of small molecule drugs, can solve problems such as adverse reactions and serious infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The preparation of formula I compound
[0073] Compounds of formula I of the present invention can be prepared by the following methods:
[0074]
[0075] Pharmaceutical compositions and methods of administration
[0076] Since the compound of the present invention has excellent JAK kinase inhibitory activity, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, and compounds containing the compound of the present invention as the main active ingredient The pharmaceutical composition of can be used to prevent and / or treat diseases related to JAK kinase activity or expression level (for example, cancer).
[0077] The pharmaceutical composition of the present invention comprises the compound of the present invention and pharmaceutically acceptable excipients or carriers in a safe and effective amount range. Wherein, "safe and effective dose" refers to: the amount of the comp...
Embodiment 1
[0090] Example 1: N-(3-methoxy-1-methyl-1H-pyrazol-4-yl)-3-(2-((3-methoxy-1-methyl-1H-pyridine Azol-4-yl)amino)-5-methylpyrimidin-4-yl)-1H-pyrrolo[2,3-c]pyridin-7-amine
[0091]
Embodiment 1-2
[0092] Example 1-2: 7-chloro-1-toluenesulfonyl-1H-pyrrolo[2,3-c]pyridine
[0093]
[0094] Under ice-water bath conditions, sodium hydrogen (375 mg, 9.37 mmol) was added in portions to a solution of compound 1-1 (950 mg, 6.25 mmol) in DMF (15 mL), and stirred for 20 minutes. Then p-toluenesulfonyl chloride (1.42 g, 9.37 mmol) was added to the solution in portions, and stirred at room temperature for 4 hours. The reaction was monitored by TLC and LCMS. After 1-1 disappeared, the reaction was quenched with 100 ml of water, extracted three times with ethyl acetate (50 mL*3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to give the product (1.45 g, yield 76%). MS(ESI):m / z=307[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com