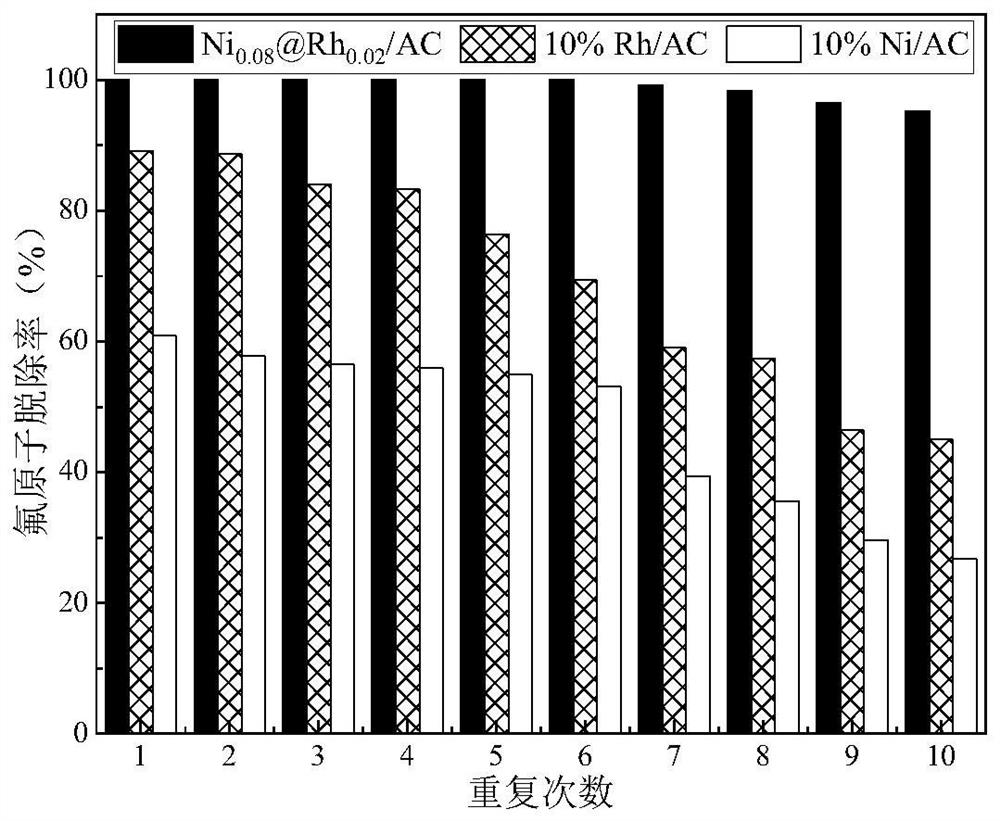
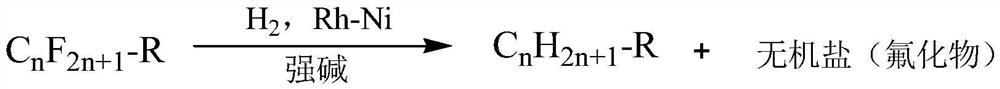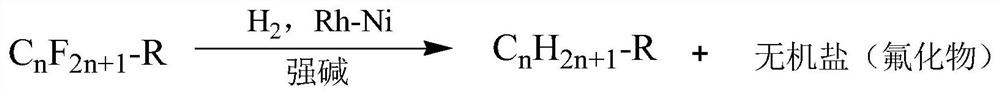A method for the efficient reductive defluorination of perfluorinated compounds by bimetallic synergistic catalysis
A perfluorinated compound, bimetal synergistic technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., can solve the problem that the catalytic activity needs to be further improved, etc. To achieve the effect of high catalytic activity and anti-poisoning performance, efficient treatment, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of heterogeneous catalytic reduction defluorination catalyst
[0027] Take a three-necked flask, add 5.21g [Ni(NH 3 ) 6 ] Cl 2 And dissolved in water, adjust the pH of the solution to be about 11.5, add pretreated activated carbon (AC) 20g to the above reaction system under magnetic stirring, continue stirring for 2.0h, add hydrazine hydrate under stirring, and continue the reaction for 1.5h, and respectively It was washed several times with distilled water and absolute ethanol, and dried in vacuum to obtain a 6% Ni / AC catalyst. Then take the three-necked flask and add 1.26g [Rh(NH 3 ) 5 ] Cl 3 And dissolved in water, adjust the pH of the solution to about 11.5, add the above-mentioned Ni / AC catalyst under magnetic stirring, continue to stir for 2.0h, add hydrazine hydrate under stirring, and continue to react for 1.5h, and wash several times with distilled water and absolute ethanol respectively , dried under vacuum to obtain Ni 0.0...
Embodiment 2
[0030] Example 2, the influence of different synergistic catalysts on the reductive defluorination of perfluorooctanoic acid
[0031] Weigh 50 mg of the catalyst prepared in Example 1, add it to a 100 mL three-necked flask, and add a concentration of 2 g / L perfluorooctanoic acid 10% 1,3-propylene glycol-water solution (V (1,3-propylene glycol) / V (water)=10 / 90) 80mL, with hydrogen as the hydrogen source (H 2 : 20mL / min), in the presence of inorganic base NaOH, the perfluorooctanoic acid in the liquid phase is carried out catalytic reduction defluorination treatment, the ratio of the amount of inorganic base NaOH to the amount of fluorine element in the reaction substrate is 1.5:1, the reaction pressure is 0.1MPa, and the reaction temperature is 45°C. See Table 2 for the specific reductive defluorination results.
[0032] Table 2 Different catalytic materials catalyze the reductive defluorination of PFOA
[0033] serial number Reaction substrate catalyst React...
Embodiment 3
[0034] Embodiment 3, different alkalis to Ni 0.08 @Rh 0.03 Effect of / AC Synergistic Catalyst on Reductive Defluorination of Perfluorooctanoic Acid
[0035] Weigh 50mg of the Ni0.08@Rh0.03 / AC synergistic catalyst prepared in Example 1, add it to a 100mL three-necked flask, and add 10% perfluorooctanoic acid-water solution (V(1 ,3-propanediol) / V (water)=10 / 90) 80mL, with hydrogen as the hydrogen source (H 2 : 20mL / min), carry out catalytic reduction defluorination treatment to perfluorooctanoic acid in the liquid phase in the presence of a strong base, the ratio of the amount of base to the amount of fluorine element in the reaction substrate is 1.5:1, and the reaction pressure is 0.1MPa , the reaction temperature is 45°C, and the specific reductive defluorination results are shown in Table 3.
[0036] Table 3 Ni in the presence of different bases 0.08 @Rh 0.03 / AC Catalyzed Heterogeneous Catalytic Reductive Defluorination of Perfluorooctanoic Acid
[0037] seri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com