Methods for treating myeloproliferative neoplasm-associated myelofibrosis and anemia
A myelofibrosis and tumor-related technology, which is applied in the field of treatment of myelofibrosis and anemia related to myeloproliferative tumors, can solve the problems of short duration of response medium, shortened splenectomy survival time, and aggravated anemia.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0192] 6.2.1 background
[0193] Myeloproliferative neoplasms (MPN)-associated myelofibrosis (MF) is a clonal myeloid neoplasm characterized by myelofibrosis, defective bone marrow function, extramedullary hematopoiesis, propensity to blastocyst transition, and inflammation (Mesa RA et al. LeukRes. 2011;35(1):12-13). Anemia and red blood cell (RBC) transfusion dependence (TD) are independent poor prognosis and survival predictors in these patients (Passamonti F et al. Blood. 2010; 115(9):1703-1708; Elena C et al. Haematologica. 2011 ; 96(1):167-170).
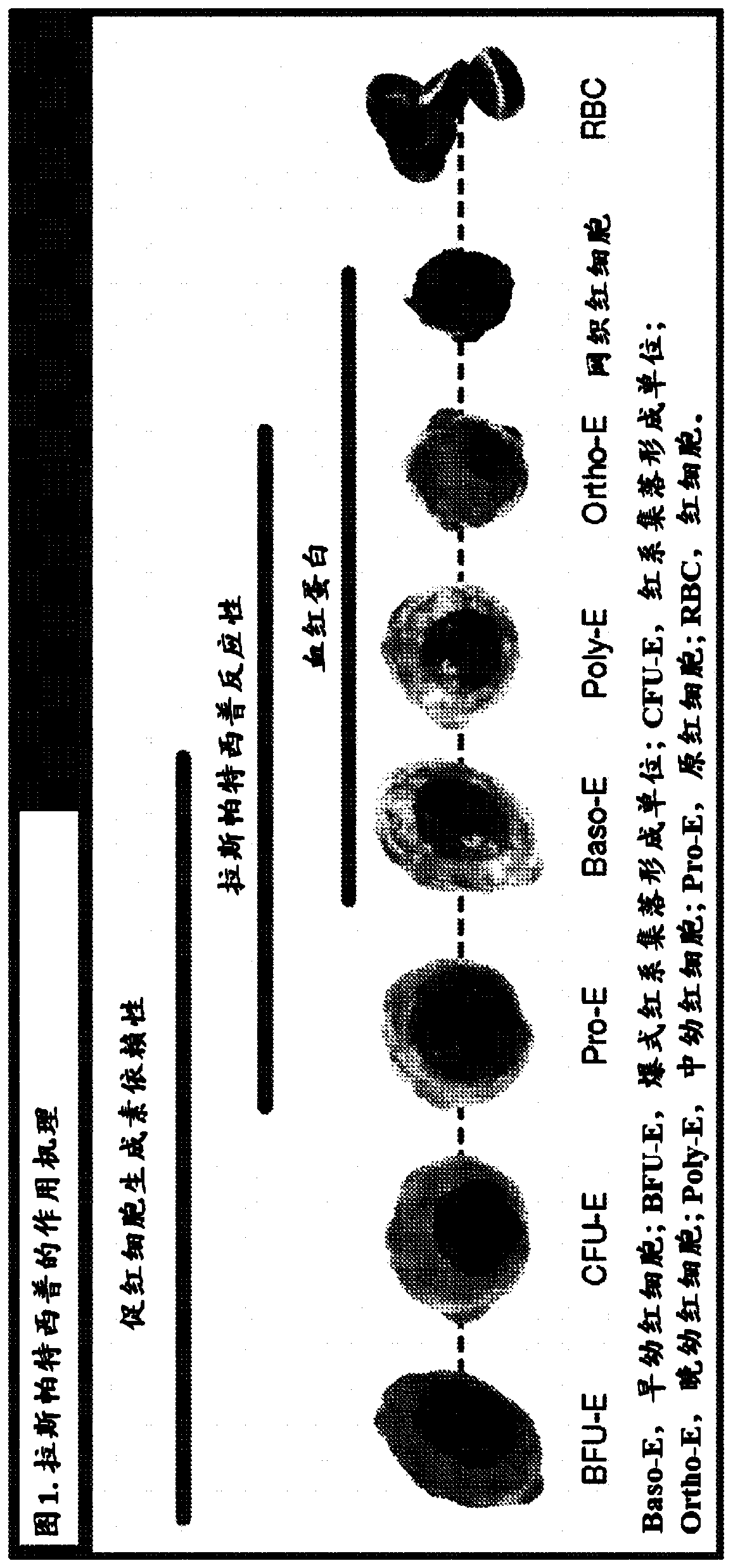
[0194] Raspartercept is a recombinant fusion protein consisting of a modified type IIB activin receptor linked to the Fc domain of human immunoglobulin G1 (IgG1) ( figure 1 ) (Attie KM et al. Am J Hematol. 2014; 89(7):766-770; Suragani RN et al. Nat Med. 2014; 20(4):408-414). Raspartercept acts as an erythroid maturation agent by binding to specific transforming growth factor-beta (TGF-beta) superfamily ligands, such as gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com