Fibroblast growth factor receptor 2-specific peptide reagents and methods
A growth factor receptor and fibroblast technology, applied in the field of fibroblast growth factor receptor 2-specific peptide reagents, can solve the problems of no obvious advantages and low contrast.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0087] The present invention will be more fully understood by reference to the following examples, which detail exemplary embodiments of the invention.
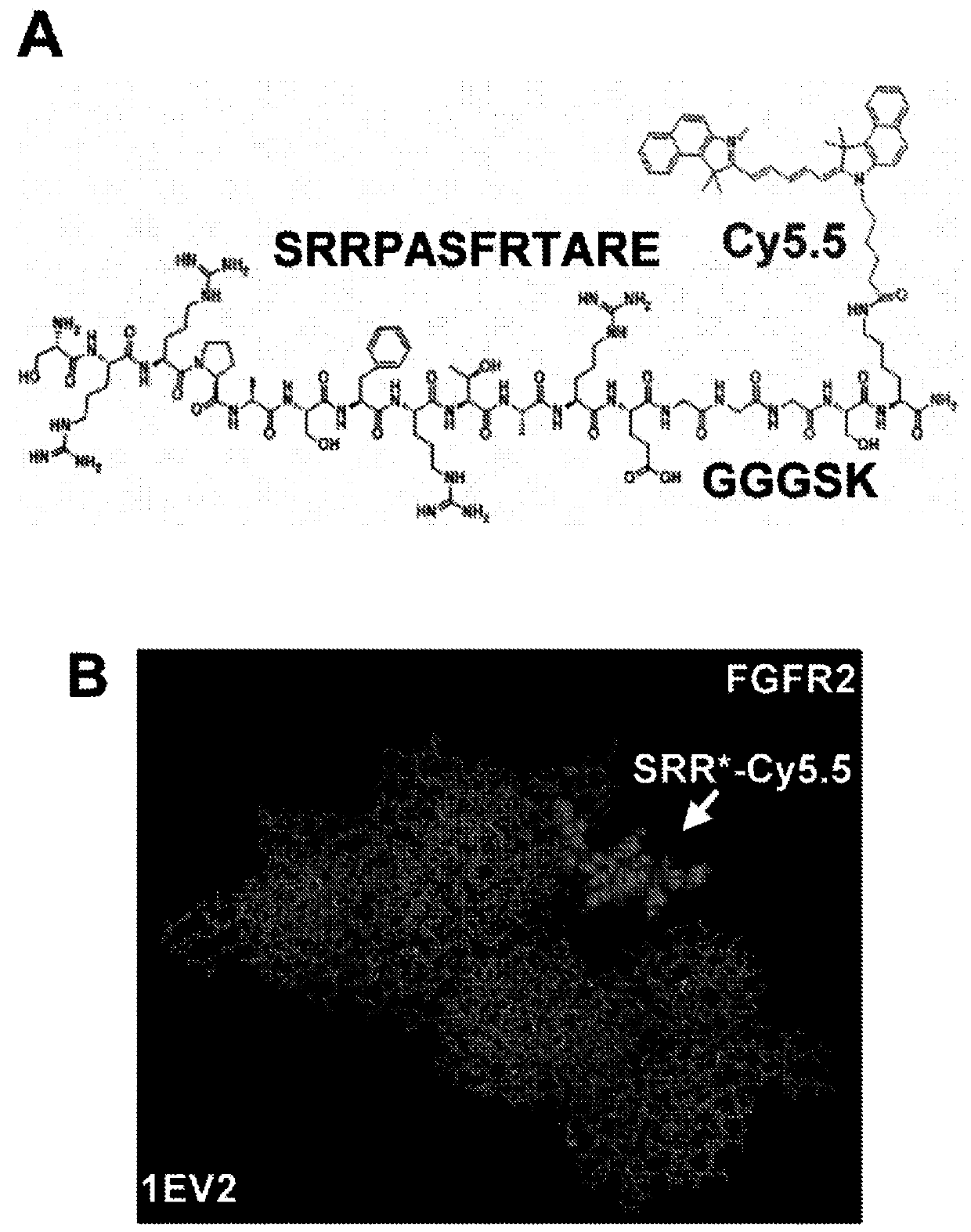
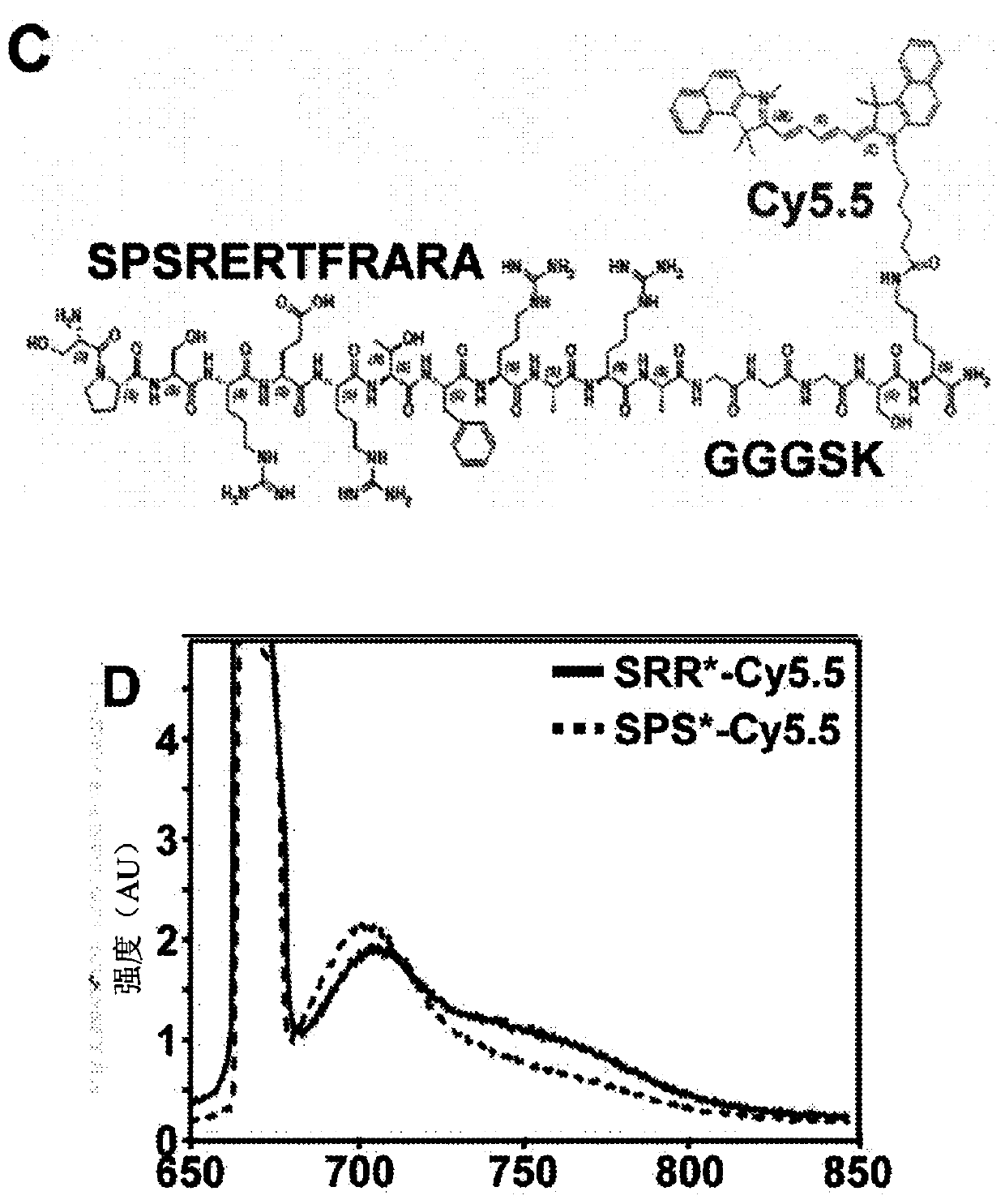
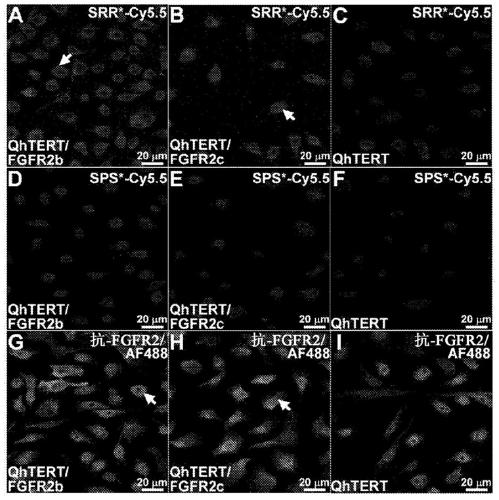
[0088]The incidence of esophageal adenocarcinoma (EAC) is rapidly increasing, and early detection of Barrett's esophagus (BE) precursor state is challenged by premalignant lesions that are difficult to detect with conventional endoscopic surveillance. Expression of fibroblast growth factor receptor 2 (FGFR2) is an early event in the development of BE to EAC and is a promising imaging target. The peptide SRRPASFRTARE that specifically binds the extracellular domain of FGFR2 was identified using phage display as described in the Examples below. This peptide was labeled with the near-infrared fluorophore Cy5.5, and specific binding to FGFR2 overexpressed in cells was confirmed in vitro. High affinity k d =67.94nM and fast binding k=0.16min -1 (6.2 minutes) was found. In human esophageal samples, peptide agents were found to ...
example 1
[0092] Expression of FGFR2
[0093] Classified by an expert gastrointestinal pathologist (HDA) including squamous (SQ), Barrett's esophagus (BE), low-grade dysplasia (LGD), high-grade dysplasia (HGD) and esophageal adenocarcinoma (EAC ) human esophageal samples were subjected to immunohistochemistry (IHC) to demonstrate representative levels of FGFR2 expression, Figure 6 .
example 2
[0095] FGFR2-specific peptides
[0096] Identification of FGFR2-specific peptides using phage display technology.
[0097] The extracellular domain (ECD) of FGFR2 consists of a signal peptide (SP) and three extracellular immunoglobulin-like domains (D1-D3), Figure 7 a. Peptide selection was performed using the extracellular domain (ECD) of FGFR2c. This area of the target is easy to image. After removing the signal peptide (#10824-H08H-50, Sino Biological), a recombinant FGFR2-ECD (Met1-Glu377) consisting of 367 amino acids was obtained. 1 μg of FGFR2-ECD was subjected to SDS-PAGE using 0.25, 0.5 and 1 μg of BSA as controls to assess quality and quantity. FGFR2-ECD with HPLC purity >97% was used. SDS-PAGE showed an apparent molecular weight of about 65-75 kDa, Figure 7 b. This result is slightly higher than the expected value of 41 kDa due to glycosylation.
[0098] Peptide selection was performed using a phage display library (New England Biolabs, Ph.D.-12) followi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com