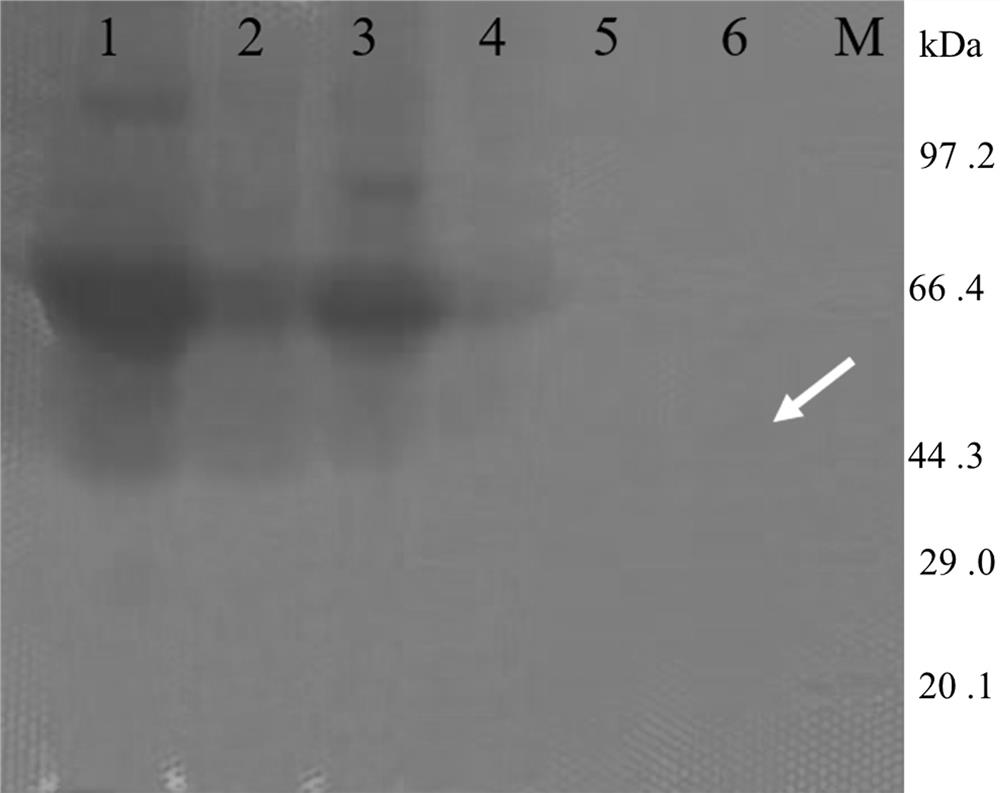
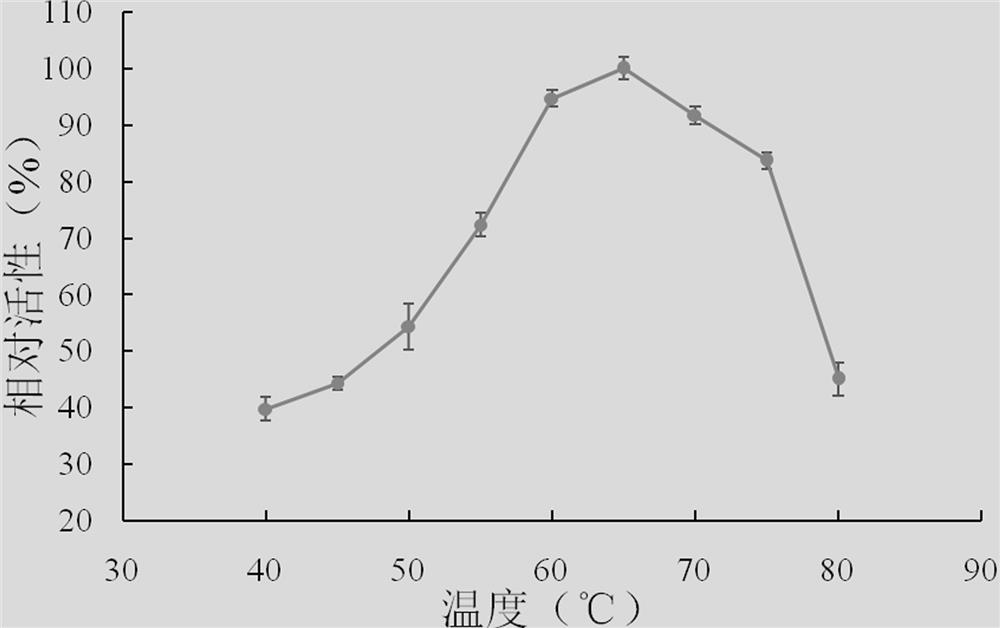
Xylanase, gene and its application
A kind of technology of gene and application, applied in the field of referring to xylanase, xylanase, gene and its application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1, the acquisition of β-mannanase Xyl1 and its coding gene
[0081] Acquisition and assembly of short sequences of microbial communities in paper mill wastewater obtained by Illumina high-throughput sequencing technology
[0082] Get 2ml paper mill waste water, get the precipitate after centrifugation and wash with 2ml PBS buffer solution (137mmol l -1 NaCl, 2.7mmoll - 1 KCl, 1.5mmol l -1 K H 2 PO 4 , 8.1mmol l -1 Na 2 HPO 4 , pH7.4) washed 3 times. DNA extraction was performed using DNeasy PowerSoilPro Kit (Qiagen, Germany). After that, high-throughput sequencing was performed on the extracted paper mill wastewater DNA using Illumina high-throughput technology, and a total of 53,886,441 short metagenomic sequences were obtained, with an average length of 148.14bp. The basic analysis process of metagenomic sequencing data analysis includes: raw data quality assessment, quality control, removal of host sequences (definite host information is required)...
Embodiment 2
[0083] Example 2, expression of xyl1 in Escherichia coli
[0084] 1. Construction of recombinant expression vector in host Escherichia coli
[0085] The ORF coding gene (SEQ ID NO.1, encoding the amino acid shown in SEQ ID No. 2) of mannanase gene xyl1 was cloned by PCR using paper mill wastewater DNA as a template, and the forward primer used was: 5' GCCTGGTGCCGCGCGGCAGCATGATTAAATTAGGATGTATCAAAGGTC3' (SEQ ID NO.5), its 5' end is added with the recombination sequence of pET28 vector: GCCTGGTGCCGCGCGGCAGC; the reverse primer is 5'TCATTTATTCATAAGGTCTATCACACTTTATTCATAAGGTCTATCACAC3' (SEQ ID NO.6), its 5' end is added with the recombination sequence of pET28 vector: TCATTTATTCATAAGGTCTATCACAC.
[0086] The purified DNA fragment of the PCR product and the vector pET28 recovered after double digestion with Cla I and Xba I were obtained by using the one-step homologous recombination enzyme Hieff CloneTM Plus Multi One StepCloning kit from Shanghai Yisheng Company. Expression vector ...
Embodiment 3
[0094] Example 3, Analysis of the Enzymatic Properties of Recombinant Xyl1 Protein
[0095] The determination of the enzymatic activity of xylanase adopts DNS method, and concrete operation is as follows:
[0096] (1) DNS configuration
[0097] Weigh 10 grams of NaOH and dissolve in approximately 400ml ddH 2 O, then weigh 10g dinitrosalicylic acid, 2g phenol, 0.5g anhydrous sodium sulfite, 200g potassium sodium tartrate tetrahydrate, dissolve them in about 300ml ddH 2 O, mix the two solutions, dilute to 1 liter, and store in the dark.
[0098] (2) Standard curve preparation
[0099] Take 9 thin-walled centrifuge tubes and add the solution according to Table 3.
[0100] table 3
[0101] Standard No. 1 2 3 4 Total xylose (μg) 0 10 20 30 Xylose volume (µL) 0 1 2 3 Add pure water (µL) 100 99 98 97
[0102] The xylose concentration was 10 mg / ml. Add 100µL of DNS to each standard sample in the above table, develop color in a boilin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com