Methods for genetic engineering kluyveromyces host cells
A technology of Kluyveromyces and host cells, applied in the field of genetic engineering Kluyveromyces host cells, which can solve the surge in demand for monoclonal antibodies, long timeline for new antibodies, and CHO cell line productivity is unlikely to be significantly improved And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
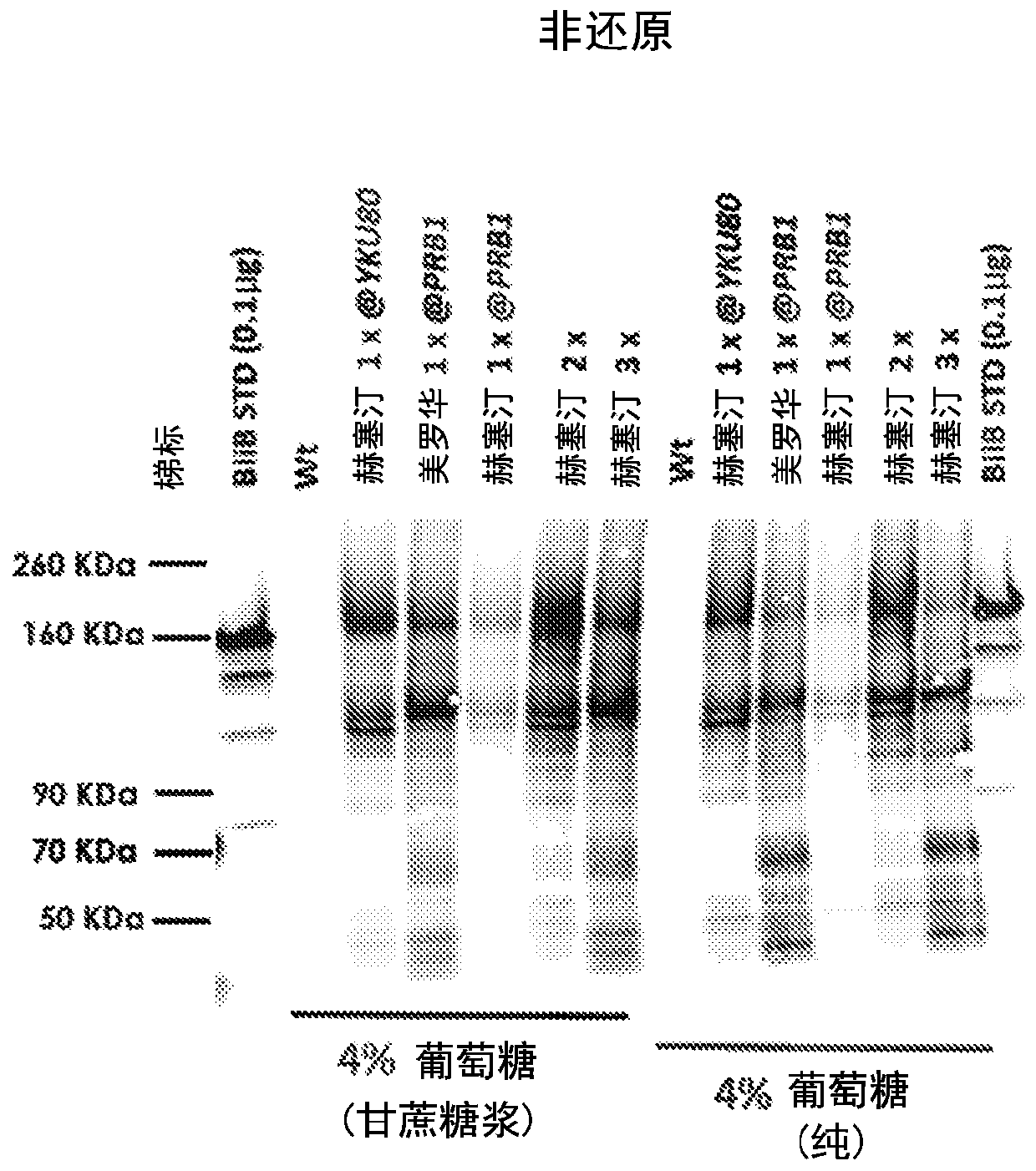
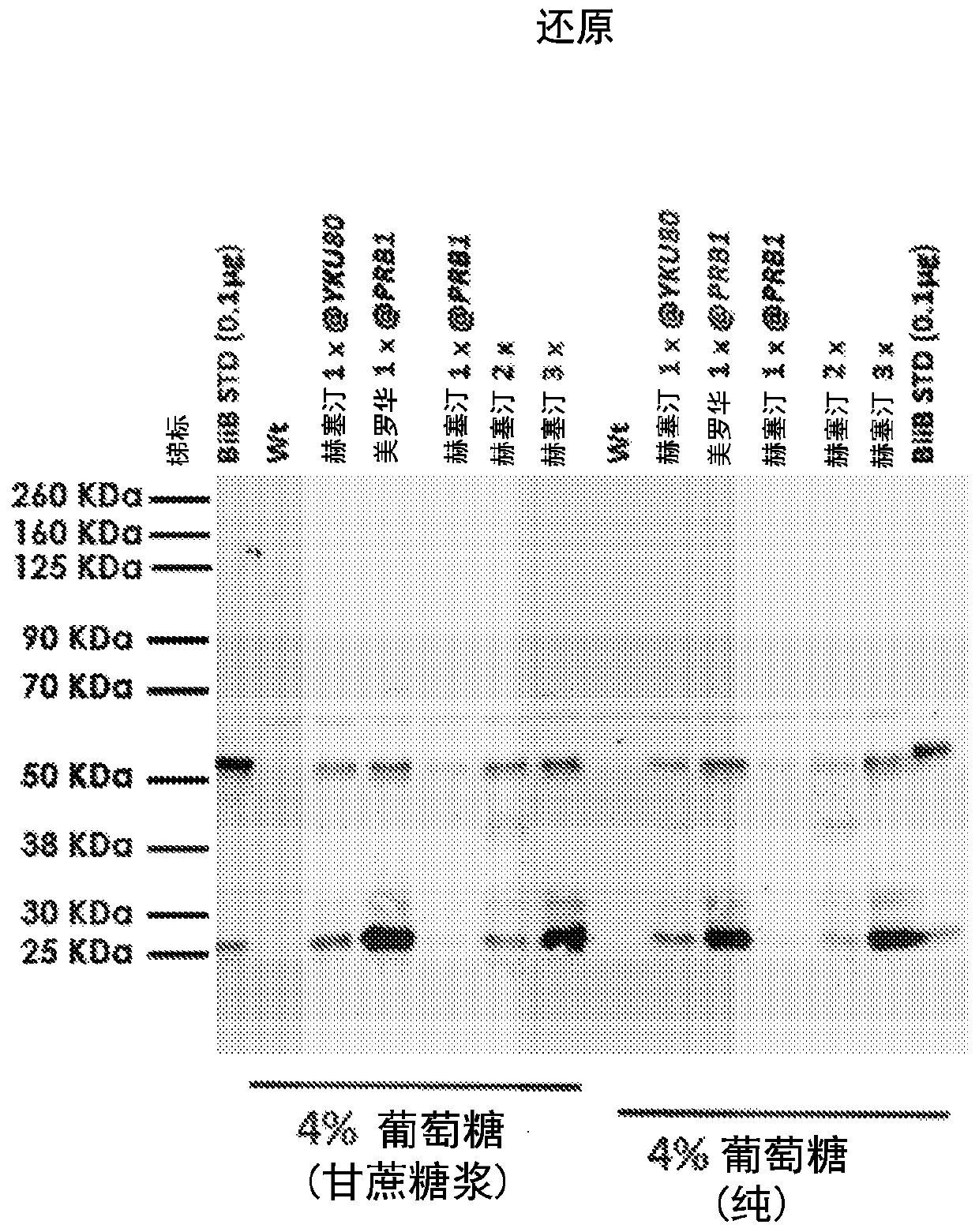
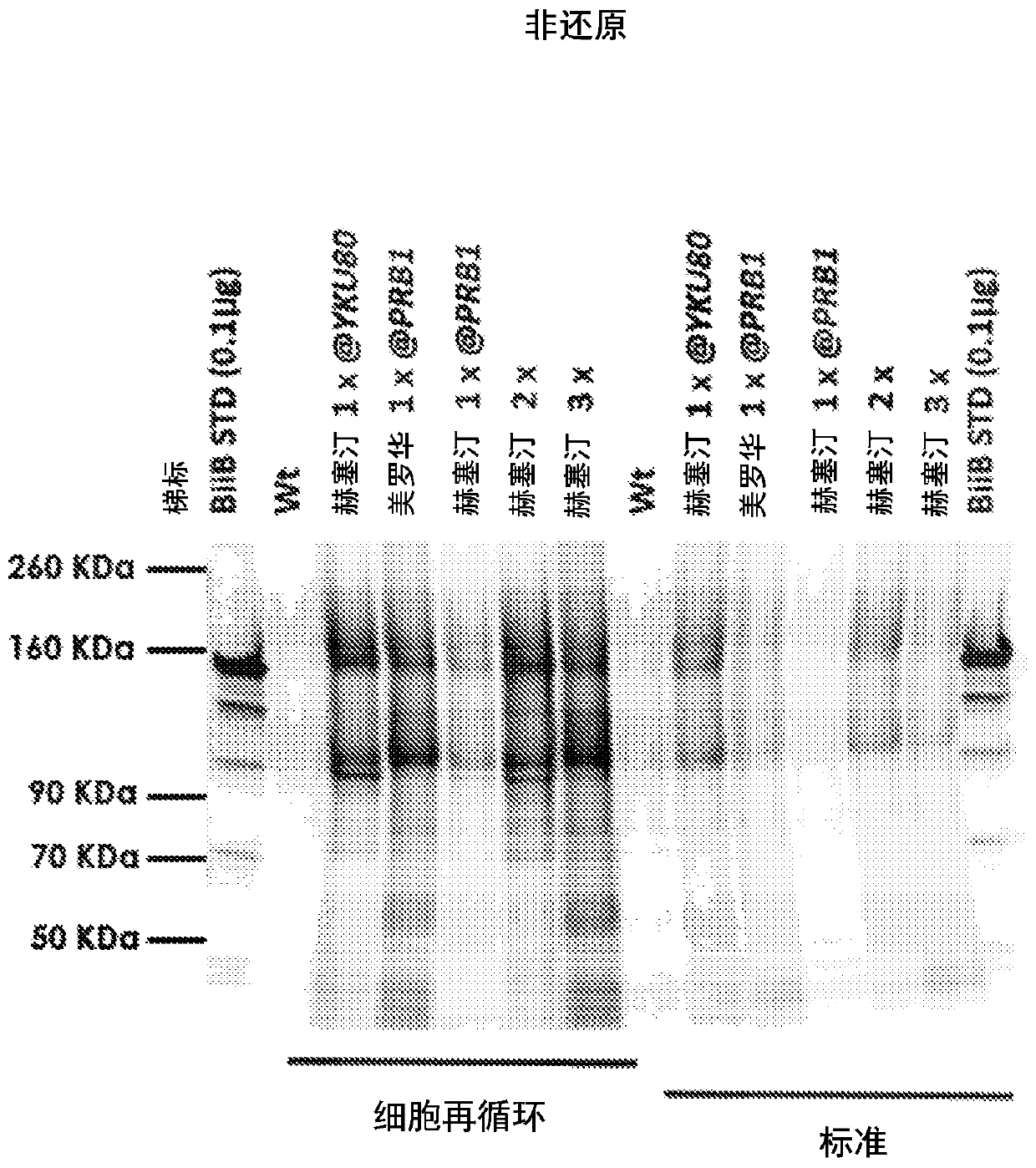
[0118] In the present invention, secretion of two full-length antibodies at high titers (≥19 μg / mL) in Kluyveromyces marxe: Herceptin (trastuzumab) and MabThera (rituximab) has been achieved . High titers were achieved using codon optimization of the amino acid sequences of the heavy chain, light chain and secretion tag according to K. marx preferred codons. The DNA expression constructs were cloned as convergent split cassettes at the same locus under a strong constitutive native glycolytic promoter. The copy number of the cassette (2 / 3 copies seemed optimal) was variable. Using 4% glucose (from sugarcane source), cell recycle treatment significantly improved titers. In addition, a K. marikus production strain with three copy numbers of Herceptin was run in a 0.5L fermenter and high titers of full-length antibodies were obtained (estimated titer ≥ 30 μg / mL). Strains were constructed using CRISPR tools with deletion of the NHEJ pathway. The copy number of gene integration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com