Drugs and compositions for ocular delivery
A composition and compound technology, applied in drug delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc.
Pending Publication Date: 2020-07-28
GRAYBUG VISION INC
View PDF58 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Unfortunately, sunitinib has been observed in both clinical trials and post-marketing clinical use
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0653] Example 1. Non-limiting examples of compounds of formula I
[0654]
Embodiment 2
[0655] Example 2A. Non-limiting Examples of Compounds of Formula II
[0656]
[0657]
[0658]
Embodiment 2B
[0659] Example 2B. Non-limiting Examples of Compounds of Formula II
[0660]
[0661]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
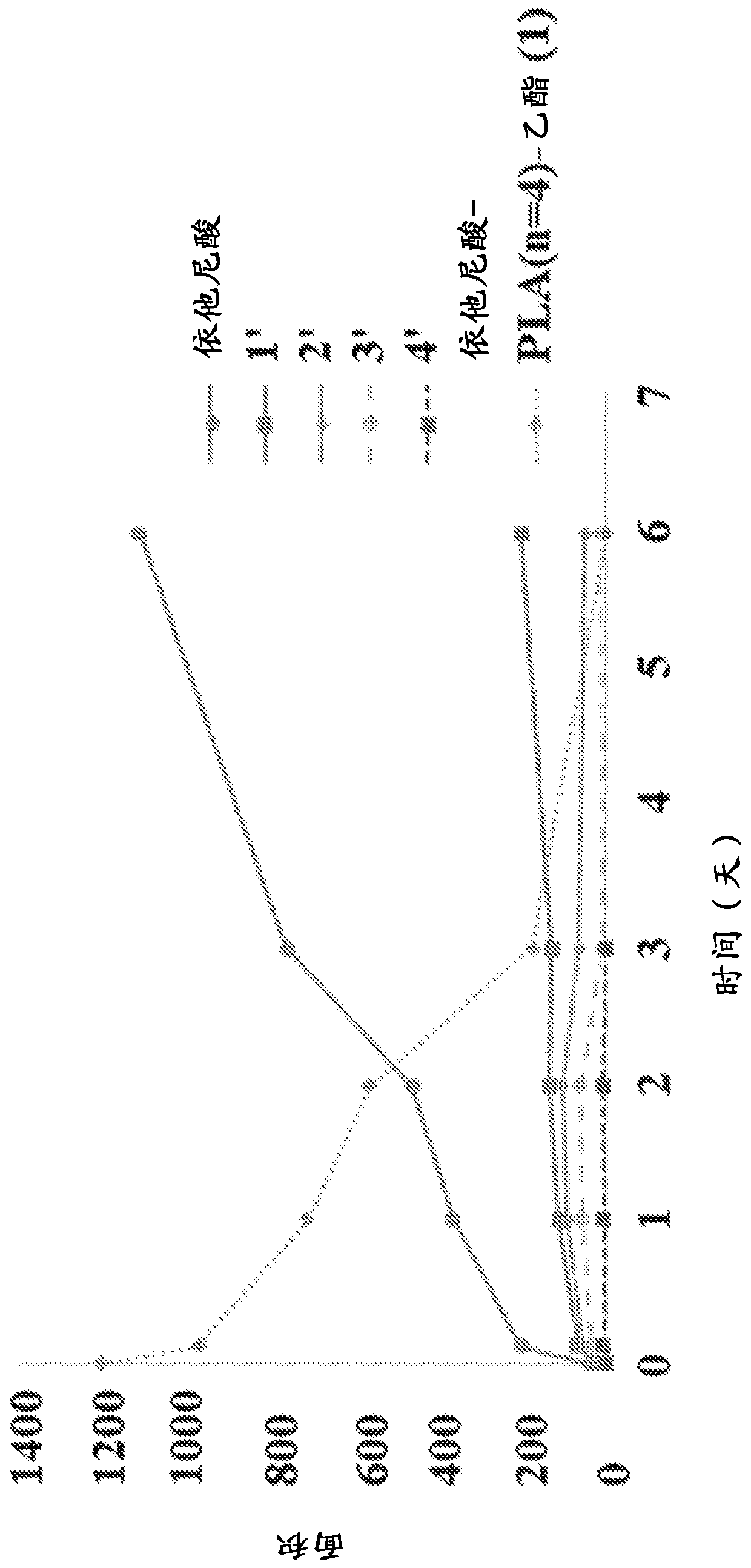
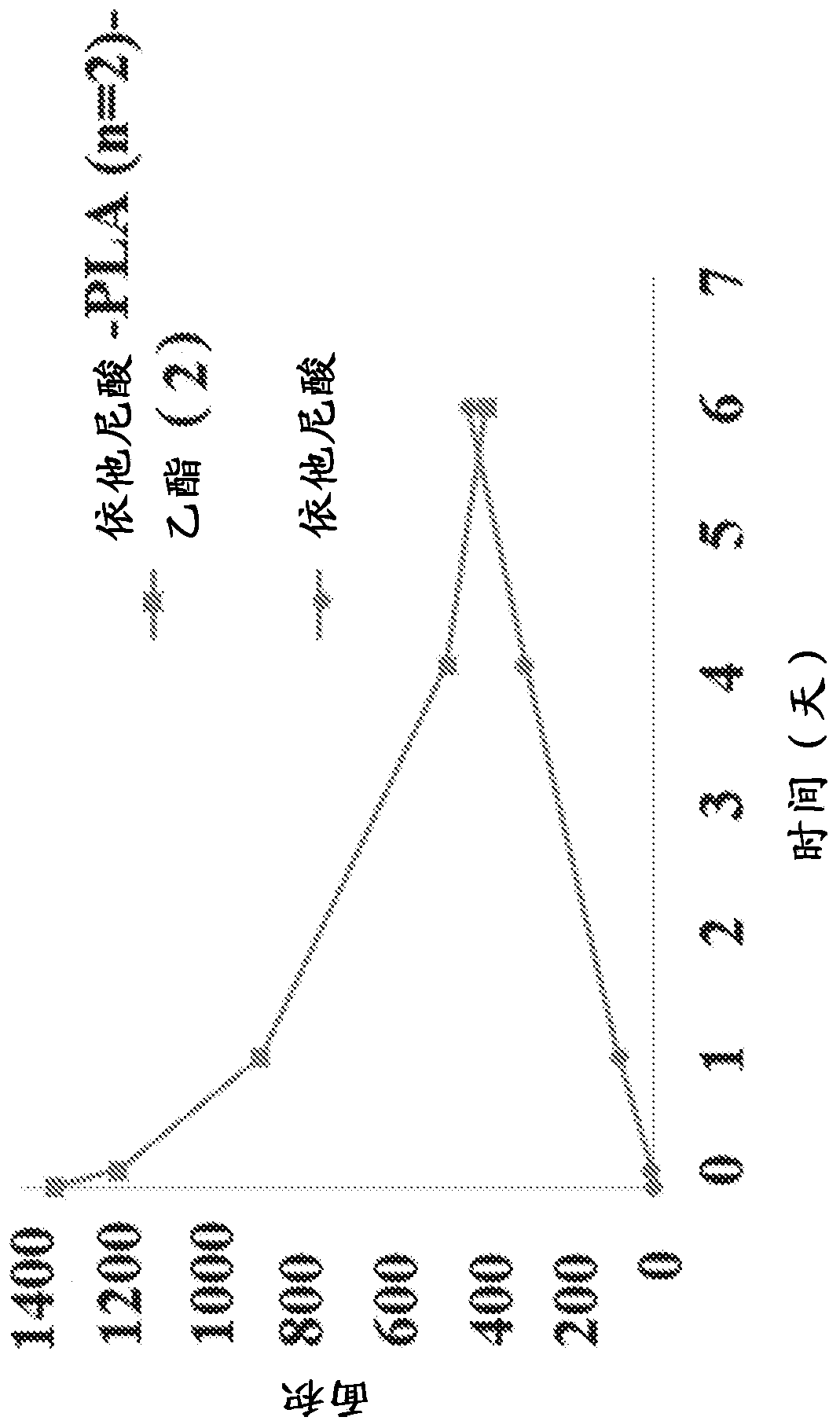
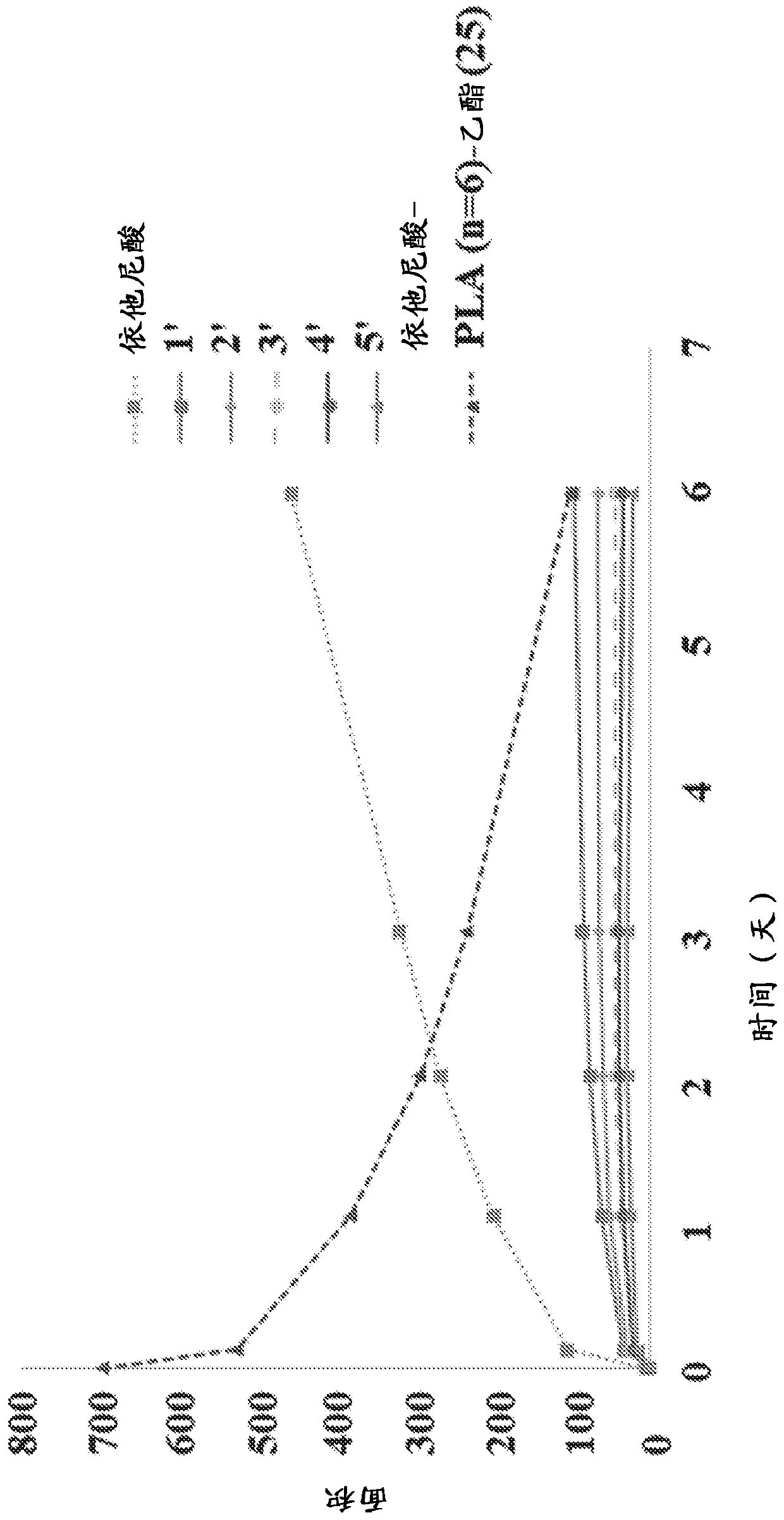
New prodrugs of therapeutically active compounds, including oligomeric prodrugs of ethacrynic acid, and compositions to treat medical disorders, for example glaucoma, a disorder or abnormality relatedto an increase in intraocular pressure (IOP), a disorder requiring neuro-protection, age-related macular degeneration, or diabetic retinopathy. Also a method for the controlled administration of timolol to a patient in need thereof, such as a human, comprising administering a prodrug of timolol in a micro-particle in vivo, wherein the timolol prodrug containing micro-particle exhibits in vitro drug release kinetics in an aqueous solution at a pH between 6-8 at body temperature of a substantially consistent release of at least 60% timolol itself by molar ratio to the prodrug of timolol or an intermediate metabolite thereof over at least 100 days.
Description
[0001] Cross References to Related Applications [0002] This application claims the benefit of U.S. Provisional Application No. 62 / 598,943, filed December 14, 2017, and U.S. Provisional Application No. 62 / 663,134, filed April 26, 2018. The entire contents of these applications are hereby incorporated by reference for all purposes. Background technique [0003] The eye is a complex organ with unique anatomy and physiology. The structure of the eye can be divided into two parts, the front and the back. The cornea, conjunctiva, aqueous humor, iris, ciliary body, and lens are located anteriorly. The posterior part includes the sclera, choroid, retinal pigment epithelium, neural retina, optic nerve, and vitreous humor. The most prevalent diseases affecting the back of the eye are age-related macular degeneration (AMD) and diabetic retinopathy, both dry and wet. The most important diseases affecting the front include glaucoma, allergic conjunctivitis, anterior uveitis, and cat...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/225C07C69/738
CPCC07C69/738C07D285/10C07D403/12C07D417/14C07D495/04C07D403/06A61K9/0019C07D417/04
Inventor J·L·克兰德杨明J·G·鲍曼N·霍安格J·齐泽姆
Owner GRAYBUG VISION INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com