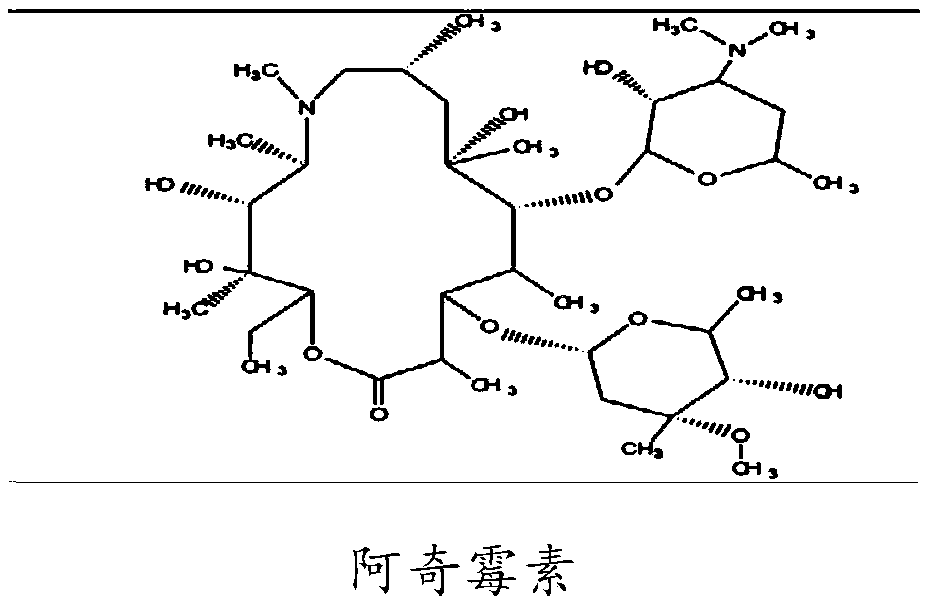
Azithromycin pharmaceutical composition, and preparation method and application of azithromycin pharmaceutical composition
A technology for azithromycin and composition, applied in the field of azithromycin composition and preparation thereof, can solve problems such as unfavorable drug quality control, complex granulation process parameters, uneven granules, etc., and achieves the consistency and reliability of curative effect, high blood drug concentration and the like. Good consistency and small clinical individual differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0054] The preparation of the azithromycin compositions of different particle sizes of embodiment 1-3
[0055] The particle diameter of the azithromycin bulk drug of Examples 1-3 is shown in Table 2.
[0056] The particle size determination method comprises the following steps: with reference to the particle size distribution determination method ("Chinese Pharmacopoeia" 2015 edition four general rules 0982 third method dry method measurement) (Malvern Mastersizer 3000 or a laser particle size analyzer with comparable performance), an appropriate amount of this product is taken, Make the shading rate of the detector between 0.5%-15%, the gas source pressure is 0.5bar, the sampling speed is 50%, the Venturi tube type is standard, the hopper gap is 1.0mm, check according to the law, and take 3 consecutive measurements average value.
[0057] Table 2
[0058] parameter Comparative example 1 Example 1 Example 2 Implementation 3 Dv(10) / μm 75.3 75.3 57.1 ...
Embodiment 4
[0060] The influence of embodiment 4 different preparation processes on the particle size of azithromycin
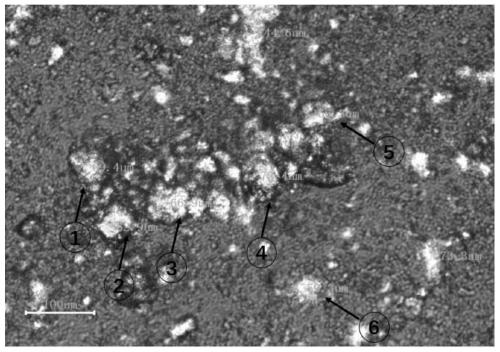
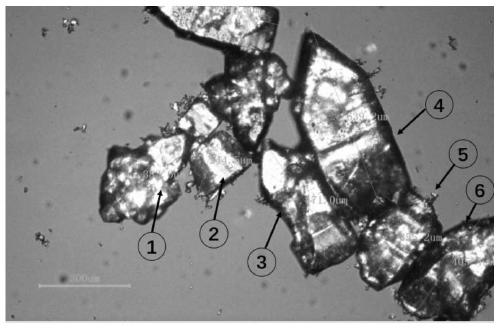
[0061] Detection method: After the azithromycin composition of comparative example 1 and embodiment 1 is dispersed in water with its insoluble medium, it is placed under a polarizing microscope (59XF, Shanghai Optical Instrument Factory) for detection, and the transmitted light is 50 times, and the azithromycin is observed in different samples. particle size.
[0062] The measurement results are shown in Table 3 and figure 1 and figure 2 .
[0063] table 3
[0064] Particle size (μm) ① ② ③ ④ ⑤ ⑥ Comparative example 1 69.4 62.9 66.9 76.4 66.3 59.3 Example 1 382.6 244.5 471.0 639.2 355.2 405.4
[0065] It can be seen from the polarizing microscope pictures that after the azithromycin API is dispersed in water in an insoluble medium, it can be seen that the particle size of the API is between 350 μm and 600 μm. After dry granul...
Embodiment 5
[0066] Example 5 Azithromycin Particle Size Effects on Blood Drug Concentration
[0067] 12 cases / group of healthy adult subjects were selected, half male and half male, and a randomized, open-label, single-dose experiment was adopted to monitor for 72 hours (T=72 hours). Table 4 shows the pharmacokinetic parameters of azithromycin in plasma after the subjects took the test preparations of azithromycin capsules of Comparative Example 1 and Examples 1-3 once orally.
[0068] Table 4
[0069] parameter (unit) Comparative example 1 Example 1 Example 2 Implementation 3 Dv(90) / μm 553 553 310 359 C max (ng / mL)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com