Pharmaceutical composition for preventing or treating cancer metastasis to lung, containing chi3l1 inhibitor as active ingredient
A composition and drug technology, applied in the directions of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., to achieve fast and efficient treatment or prevention effects, and the effects of preventing and inhibiting lung metastases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0146] Hereinafter, the present invention will be described in more detail through examples and the like, but the content and scope of the present invention should not be interpreted by narrowing or limiting the content and scope of the present invention through the following examples and the like. Moreover, based on the disclosure of the present invention including the following embodiments, it is obvious that those skilled in the art can easily implement the present invention without specific experimental results, and such variations and modifications naturally belong to the scope of the appended claims.
[0147]
[0148] 1) Animal model
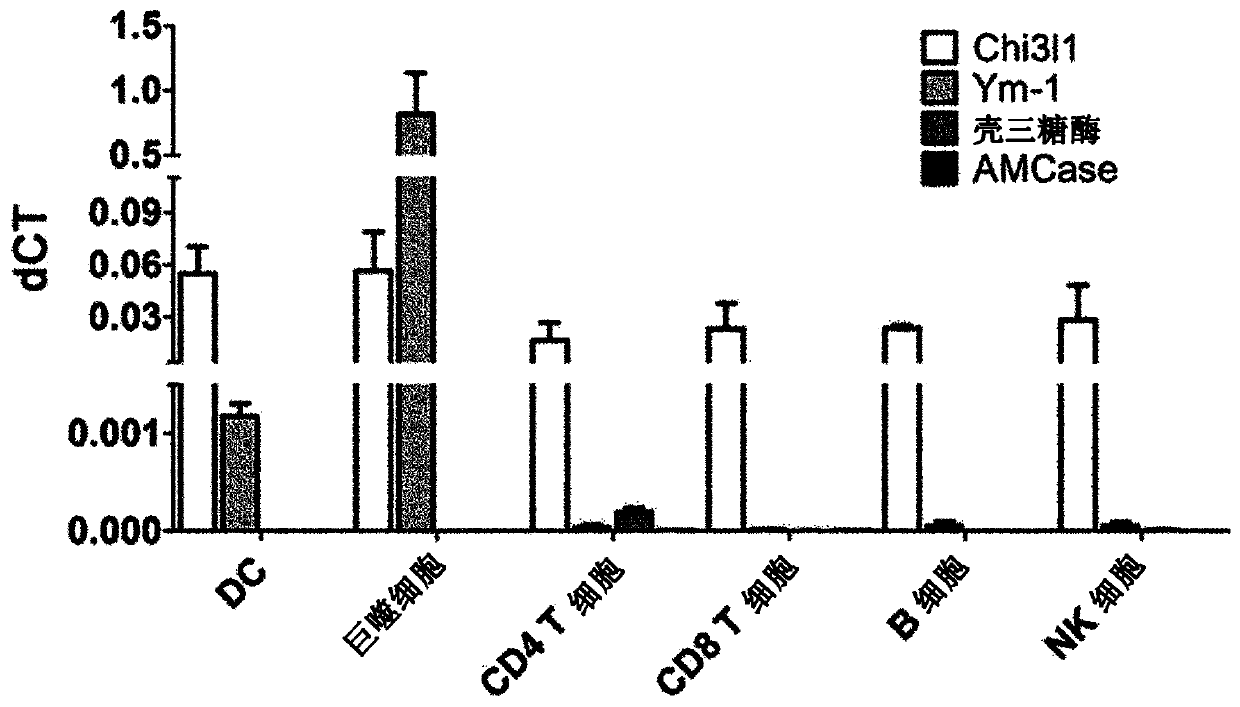
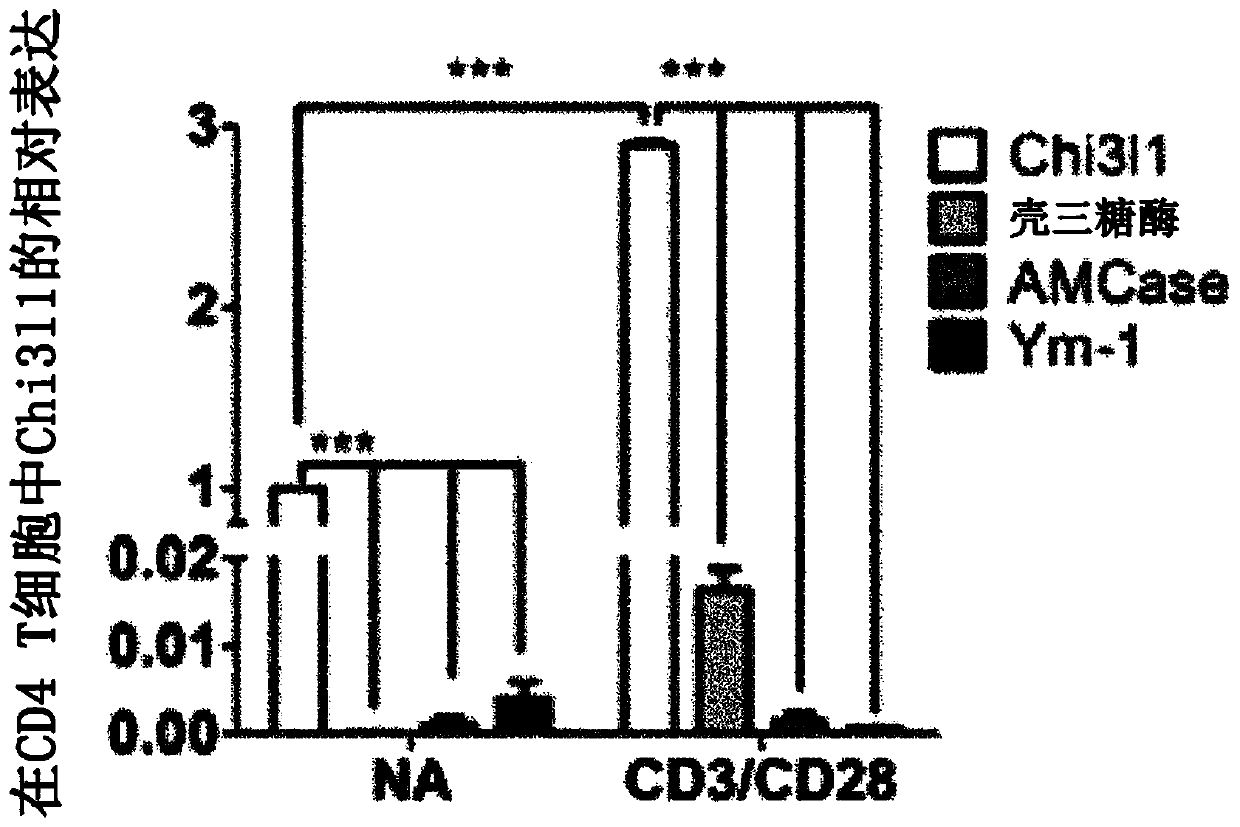
[0149] C57BL / 6J mice aged 6-8 weeks were used. Chi3l1 KO mice used were provided by Jack A Elias (Brown University). All mice were reared and kept in a specific aseptic facility (Hanyang University), maintaining the facility conditions of 21±1°C temperature, 50±5% humidity and 12-hour cycle of day and night (regular chow, PicoLab Rode...
manufacture example 1
[0180] Synthesis of dNP2-HA2 peptide and Chi3l1 siRNA
[0181] A dNP2-HA2 peptide having the Chi3l1 siRNA sequence of SEQ ID NO: 1 and the amino acid sequence of SEQ ID NO: 2 was synthesized.
[0182] Here, the siRNA of Chi3l1 represented by the sequence of SEQ ID NO: 1 (hereinafter also referred to as 'siChi3l1') and the cell-permeable peptide (hereinafter also referred to as 'dNP2') composed of the amino acid sequence of SEQ ID NO: 2 were used to perform marked.
[0183] After designing each sequence, the Chi3l1 siRNA represented by the sequence No. 1 used was synthesized and manufactured by entrusting Bioneer, and the dNP2-HA2 peptide represented by the sequence No. 2 was synthesized and manufactured by entrusting Genscript of.
[0184] serial number 1
[0185] CAGGAGUUUAAUCUCUUGCAA
[0186] serial number 2.
[0187] KIKKVKKKGRKGSKIKKVKKKGRKGLFGAIAGFIENGWEGMIDG
Embodiment 1
[0188] Production of dNP2-siChi3l1 complex.
[0189] In order to fuse the dNP2-HA peptide having the amino acid sequence of siChi3l1 of SEQ ID NO: 1 and SEQ ID NO: 2 produced in Production Example 1, 2ug of siChi3l1 was reacted with 70ug of dNP2-HA2 peptide for 30 minutes at room temperature and in the dark. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com