A kind of autofluorescence nanogel and its preparation method and application
A technology of nanogel and autofluorescence, which is applied in the application field of nanogel in the field of medicine, can solve the problems of denaturation, few therapeutic drugs targeted and efficient delivery, and lack of specificity, so as to achieve controllable preparation process, The effect of fast and efficient reaction conditions and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of Hyaluronic Acid Lysine Tetrazole Derivatives (HA-Lys-Tet)
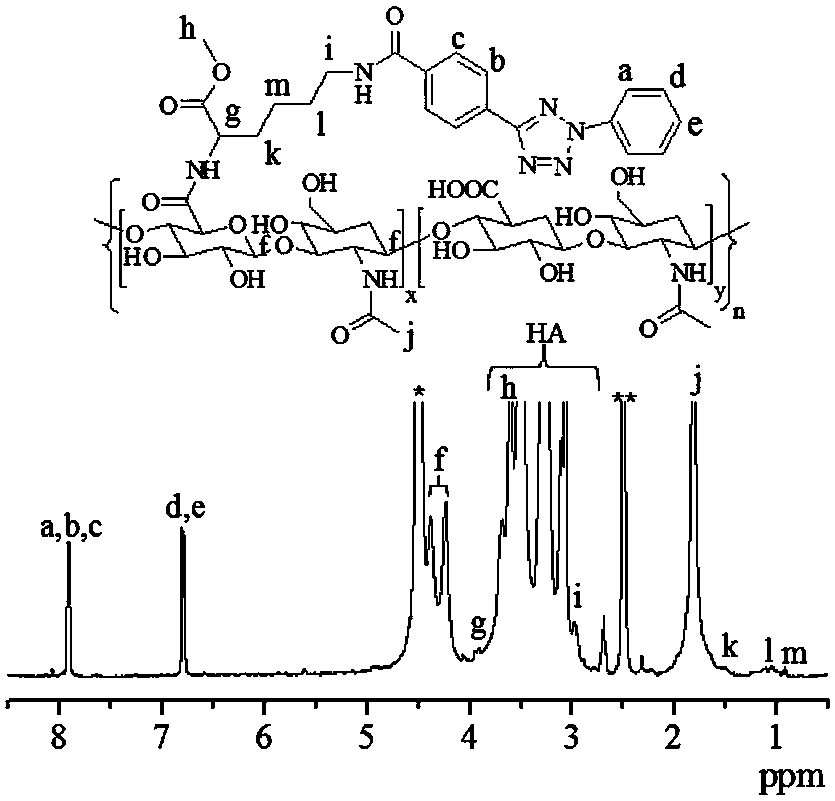
[0048] Under the condition of nitrogen protection, add tetrazole (608 mg), 10 mL of dimethyl sulfoxide, and DCC (120 mg) into a 50 mL two-necked bottle and stir for 24 hours. HA-Lys-NH 2 ( M n =35 K, 0.4 g) was dissolved in 30 mL formamide, added to the tetrazole solution after dissolving, stirred for 10 min, added DMAP (80 mg,), reacted for 48 hours, filtered, and the filtrate was mixed with water and dimethyl sulfoxide After dialysis, change to pure water dialysis, and freeze-dry to obtain the product HA-Lys-Tet (yield 69%); HA-Lys-Tet NMR characterization see figure 1 , 1 H NMR (D 2 O / DMSO-d 6 ): HA: δ 1.82, 2.70–3.68, and 4.23–4.38; Lys: δ 0.92,1.06, 1.52, 2.97, 3.61 and 3.95; Tet: δ 7.91,7.92 and 6.79, 6.80.
[0049] Four-arm polyethylene glycol tetrazole derivatives (PEG-Tet4) and chitosan tetrazole derivatives (Chit-Tet) can be prepared by replacing polymers. The structural f...
Embodiment 2
[0052] Example 2 Synthesis of Hyaluronic Acid Cystamine Methacrylate Derivatives (HA-Cy-MA)
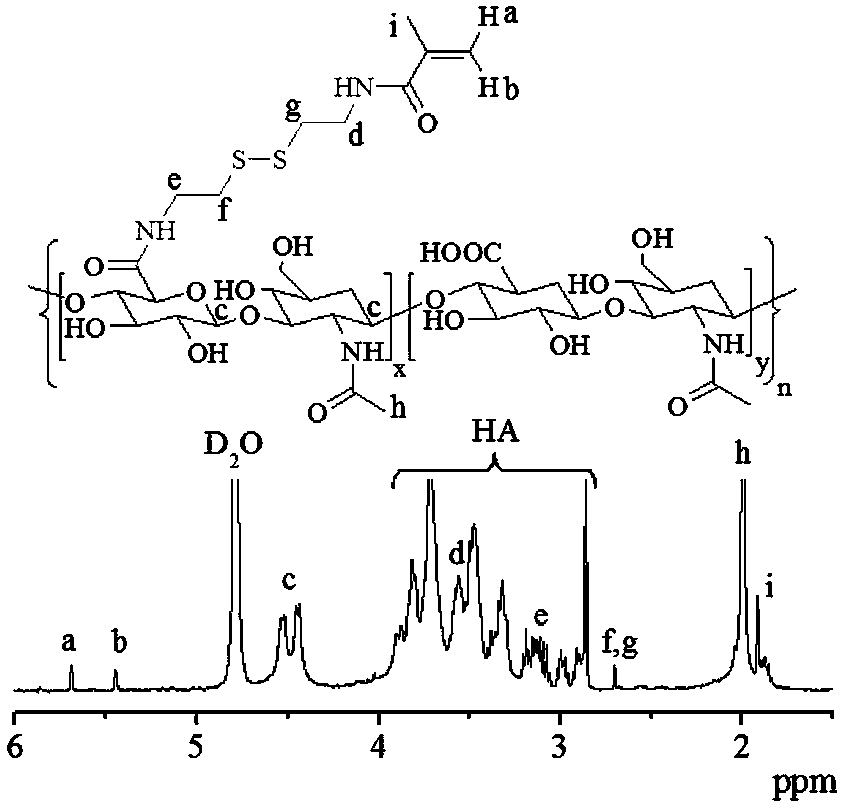
[0053] HA-Cy-MA was synthesized in two steps, first the methacrylic acid derivative of cystamine (MA-Cy-NH 2 ) by Boc-Cy-NH 2 After reacting with methacryloyl chloride, it can be obtained by removing Boc protection, and then, under the condition of nitrogen protection, MA-Cy-NH 2 (14.5 mg, 66 μmol) was added to HA (50 mg, 1.43 μmol) activated by EDC (75.9 mg, 0.396 mmol) and NHS (22.8 mg, 0.198 mmol) in 5 mL of secondary water, placed in 40 °C oil In the bath, react in the dark for 24 hours, then dialyze with water, freeze-dry, and the yield is 92%; HA-Cy-MA nuclear magnetic characterization sees attached figure 2 , 1 H NMR (D 2 O): HA: δ 2.00,2.86–3.88, and 4.44–4.52; Cys: δ 2.70, 3.11–3.15 and 3.56; MA: δ 1.92, 5.45and 5.69.
Embodiment 3
[0054] Example Synthesis of Tricystine Methacrylamide Derivatives (MA-Cys-MA)
[0055] In an ice-water bath, a solution of cystine (1.2 g, 5.0 mmol) in NaOH (1.5 M, 10 mL) was added dropwise to methacryloyl chloride (2.0 mL, 20.6 mmol) in DCM (10 mL). The reaction was carried out in an ice-water bath for 4 h, and the pH was adjusted to 9.0 with NaOH solution during the reaction. After the reaction, the aqueous layer was separated with a separatory funnel, and about 3 mL of HCl (2M) was added dropwise to it, filtered, and vacuum-dried to obtain 1.72 g of white powder with a yield of 91%. MA-Cys-MA NMR characterization see attached image 3 , 1 H NMR (400 MHz, DMSO-d 6 ): MA (δ 5.72, 5.39 and 1.85), Cys (δ 12.92, 8.24, 4.53, 3.18 and 3.03).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com