Compositions and methods for inhibiting aldh2 expression
A technology of S1-L-S2 and oligonucleotides, applied in the field of compositions and methods for inhibiting ALDH2 expression, capable of solving problems such as poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
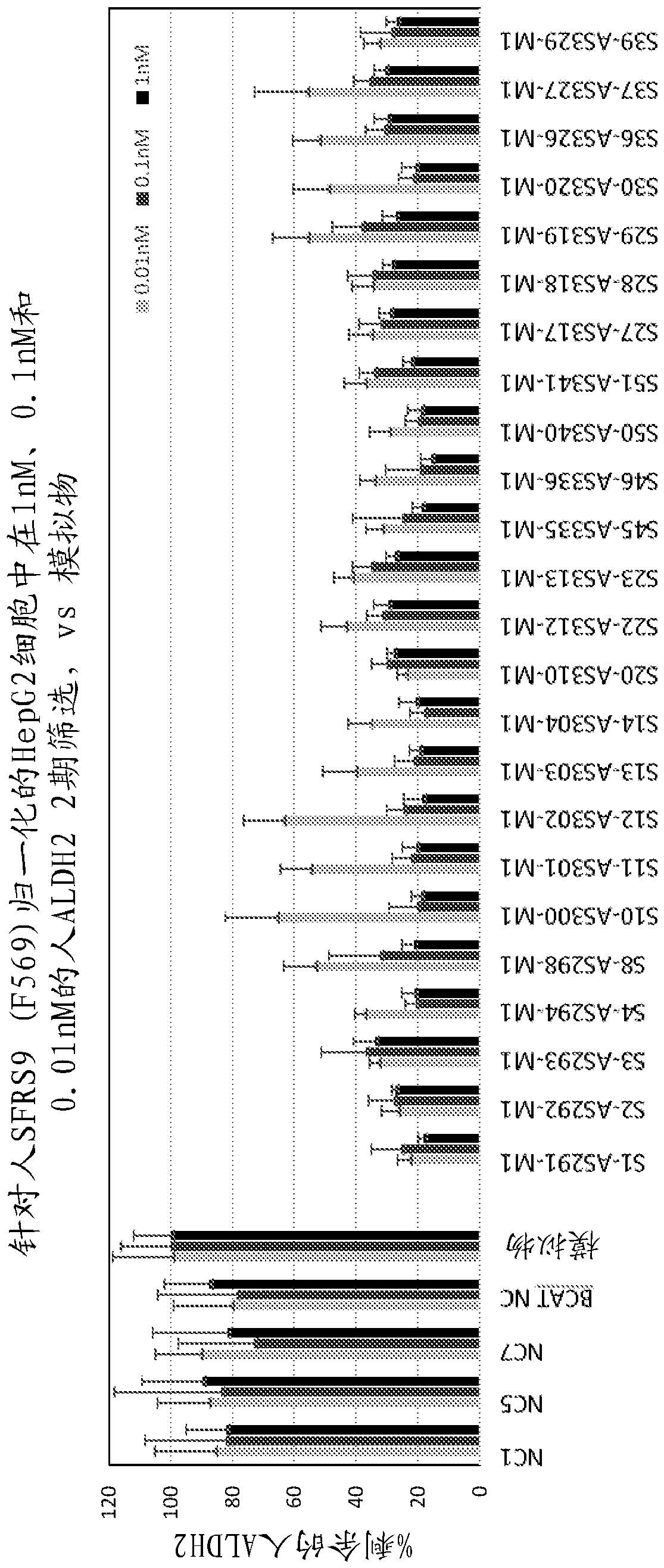
[0162] Example 1: Development of ALDH2 oligonucleotide inhibitors using human and mouse cell-based assays
[0163] figure 1 The workflow for developing candidate oligonucleotides for the inhibition of ALDH2 expression using human and mouse based assays is shown. First, a computer-based algorithm was used to generate candidate oligonucleotide sequences (25-27-mers) for ALDH2 inhibition. Cell-based assays and PCR assays were then used to assess the ability of candidate oligonucleotides to reduce ALDH2 expression.
[0164] A computer-based algorithm provided oligonucleotides complementary to human ALDH2 mRNA (SEQ ID NO:608, Table 1), some of which were also complementary to cynomolgus ALDH2 mRNA (SEQ ID NO:609, Table 1) and / or or mouse ALDH2 mRNA (SEQ ID NO:610, Table 1) complementation.
[0165] Table 1. Sequences of human, cynomolgus monkey and mouse ALDH2 mRNA
[0166] species GenBank RefSeq # SEQ ID NO. people NM_000690.3 608 cynomolgus monk...
Embodiment 2
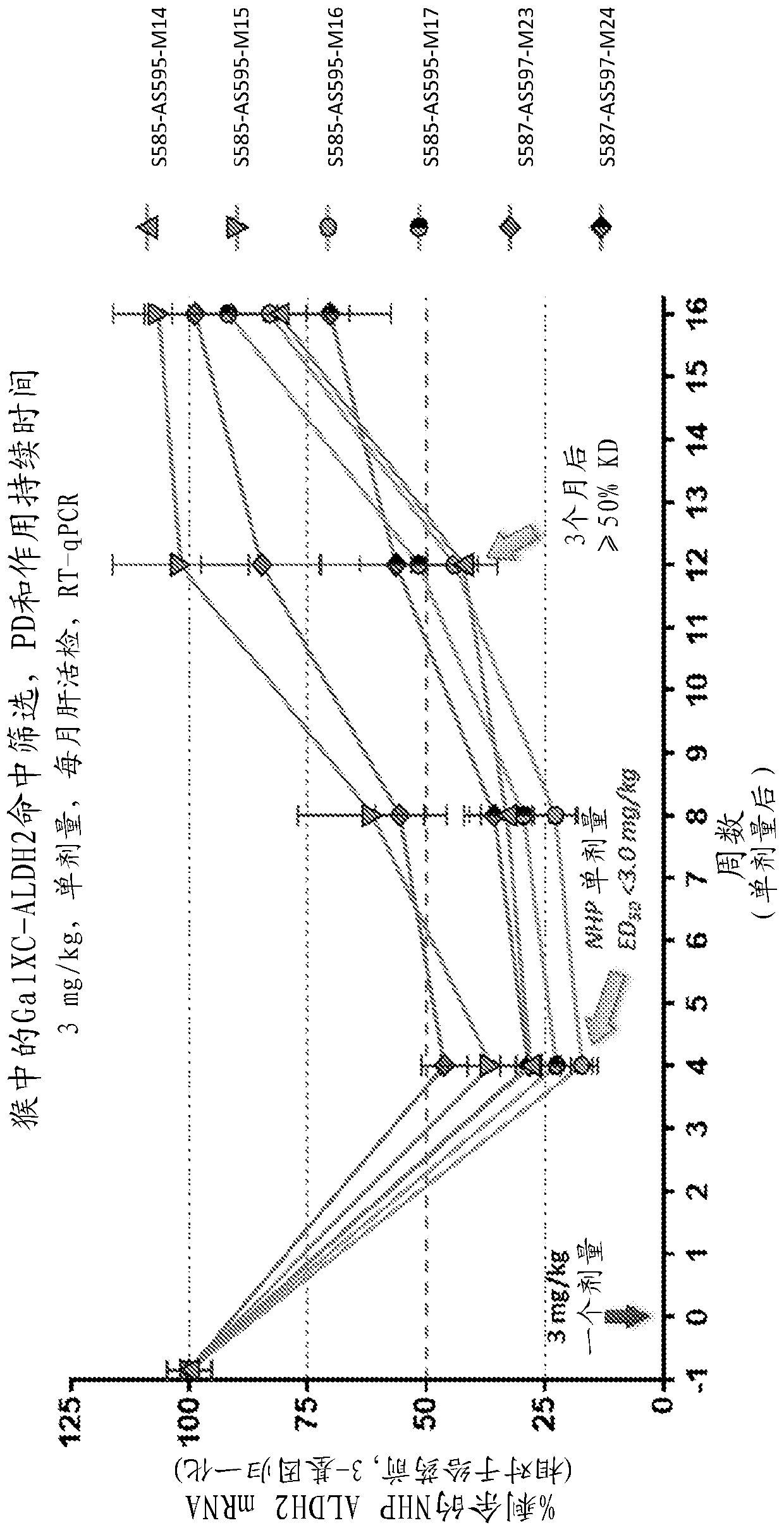
[0184] Example 2: Duration study of GalNAc-conjugated ALDH2 oligonucleotides in non-human primates (NHP) study
[0185] The study was designed to evaluate single doses of GalNAc-conjugated GGs with different modification patterns (e.g., with different numbers of 2'-fluoro modifications and / or different numbers of phosphorothioate linkages in the antisense strand). Pharmacodynamics of ALDH2 oligonucleotides. The GalNAc-conjugated ALDH2 oligonucleotides tested in this study were: S585-AS595-M14, S585-AS595-M15, S585-AS595-M16, S585-AS595-M17, S587-AS597-M23 and S587- AS597-M24. A single dose of GalNAc-conjugated ALDH2 oligonucleotide was injected subcutaneously at 3 mg / kg in non-human primates (n=4 per group). Animals were fasted overnight, and serum samples and liver biopsies were collected the next morning before feeding. One biopsy sample per dose was collected for each animal during the acclimatization period and 3 biopsies were collected at 4, 8, 12 or 16 weeks post-...
Embodiment 3
[0188] Example 3: Improvement of GalNAc-conjugated ALDH2 oligonucleotides using different modifications
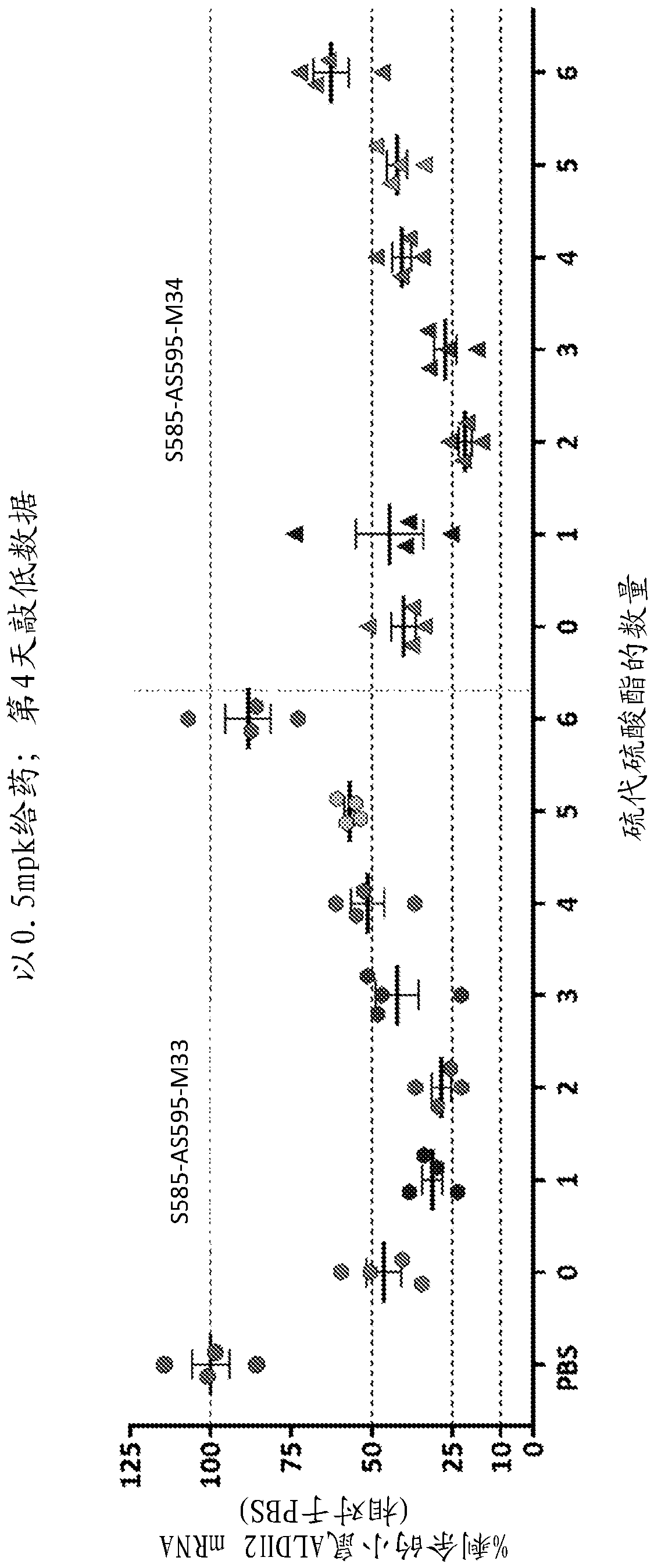
[0189] This study was designed to evaluate the effect of different modification patterns on the activity of GalNAc-conjugated ALDH2 oligonucleotides in reducing ALDH2 mRNA levels. Such as Figure 9 Two GalNAc-conjugated ALDH2 oligonucleotides (S585- AS595 and S587-AS597). Five GalNAc-conjugated ALDH2 oligonucleotides showed higher activity in reducing ALDH2 mRNA levels (S585-AS595-M23, S585-AS595-M24, S585-AS595-M16, S585-AS595-M17 and S587-AS597- M23).
[0190] Also tested for its in vivo activity in reducing ALDH2 mRNA levels in mice Figure 9 Several GalNAc-conjugated ALDH2 oligonucleotides tested in . A single dose of GalNAc-conjugated ALDH2 oligonucleotide was administered subcutaneously to mice at 0.5 mg / kg, and the level of ALDH2 mRNA in mouse liver was assessed by qPCR 4 days after administration. The results showed that the modification patterns M22, M15, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com