Attenuated live vaccine of Toxoplasma gondii lacking ap2iv-1 gene and its construction method
A technology of AP2IV-1 and Toxoplasma gondii, applied in the direction of microorganism-based methods, other methods of inserting foreign genetic materials, vaccines, etc., can solve the problems of restricting the use of vaccines, strong virulence, etc., and achieve high immune protection efficacy and pathogenicity. The effect of reducing the disease and reducing the growth rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
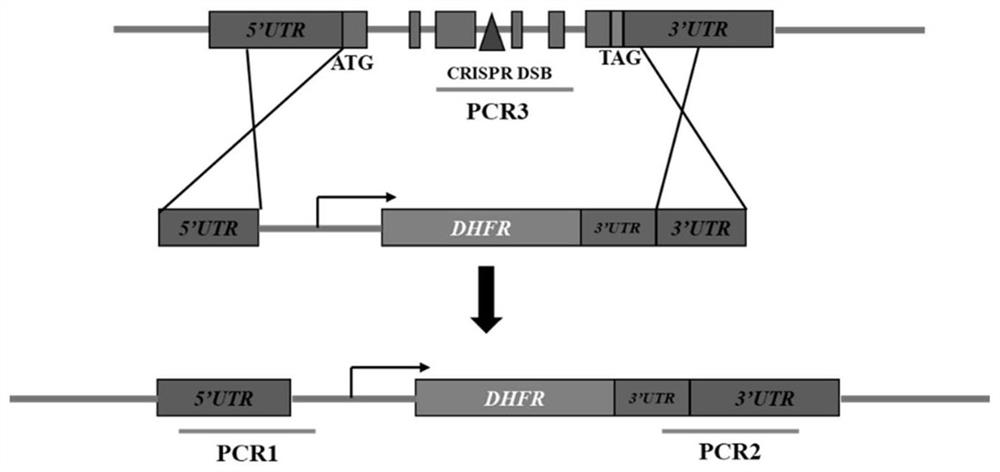
[0039] Example 1 Construction of Toxoplasma gondii AP2IV-1 gene deletion strain
[0040] The AP2IV-1 gene deletion strain was constructed with ΔKu80 RH as the base strain.
[0041] ΔKu80 RH is a type I strain of Toxoplasma gondii that loses the ability to form cysts in animals. The sequence of AP2IV-1 is conserved among different types of Toxoplasma gondii, and its amino acid sequence is shown in SEQ ID NO.1, and its nucleotide sequence is shown in SEQ ID NO.2.
[0042] 1. Construction of CRISPR / CAS9 system plasmid pSAG1-CAS9-TgU6-sgAP2IV-1
[0043] Using the pSAG1-CAS9-TgU6-sgUPRT plasmid as a template, the multi-segment seamless cloning kit ( (Seamless Cloning and Assembly Kit) replace the gRNA of UPRT gene with the gRNA of AP2IV-1 gene, the specific operation steps are as follows:
[0044] (1) Design of gRNA
[0045] The AP2IV-1 gRNA was designed in the ToxoDB (http: / / gRNA.ctegd.uga.edu / ) database of Toxoplasma gondii. Since the AP2IV-1 gene sequence is less than 2000b...
Embodiment 2
[0103] Example 2 Detection of proliferation rate, virulence and immune protection of Toxoplasma gondii AP2IV-1 gene deletion strains
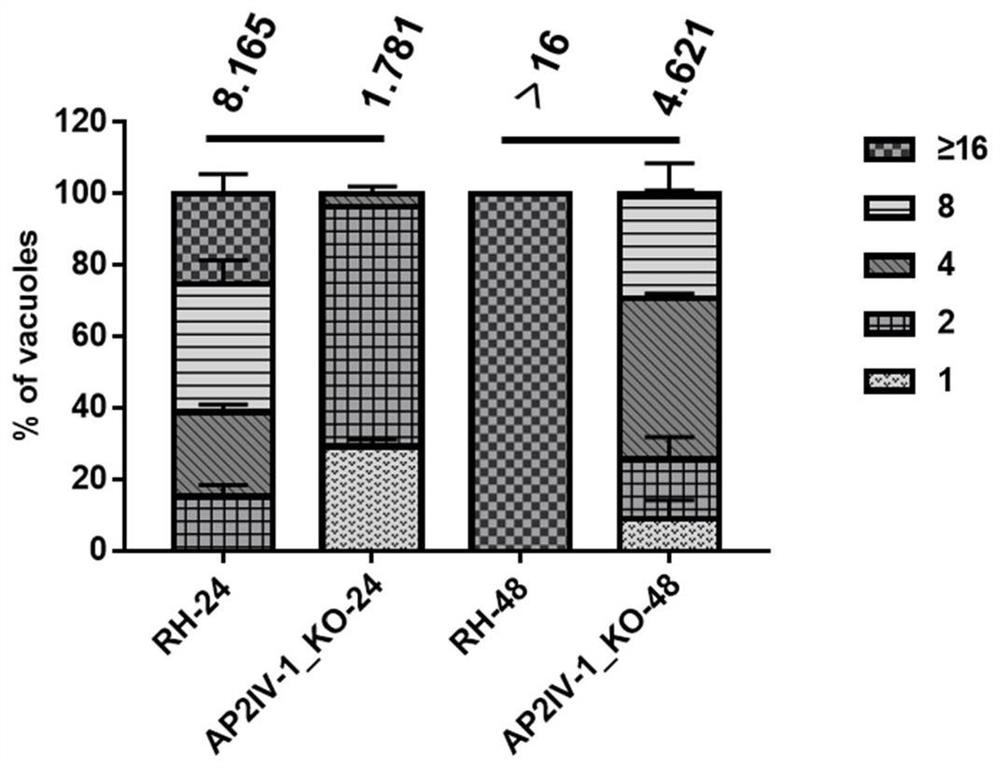
[0104] 1. In vitro proliferation experiment of RH ΔAP2IV-1 strain
[0105] The intracellular proliferation rate of the Toxoplasma gondii AP2IV-1 gene deletion strain RHΔAP2IV-1 constructed in Example 1 was detected, and the specific method was as follows:
[0106] Tachyzoites of freshly released ΔKu80 RH and RH ΔAP2IV-1 strains were collected and inoculated with 10 5 Tachyzoites were placed in a 12-well plate confluent with HFF cells (human foreskin fibroblasts, purchased from ATCC Company) (a sterile cell slide was placed before the cells were plated). After 1 h of inoculation, the non-invading worms were washed away, and the culture was continued in the incubator. After culturing for 24h or 48h, carry out the IFA test, the specific method is as follows:
[0107] ① Fix the cells infected with Toxoplasma gondii in 4% paraformaldehyde at 37°C...
Embodiment 3
[0129] Example 3 Humoral immunity and cellular immune response monitoring of host immunized with RHΔAP2IV-1 strain
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com