Poly-R-indole blue light absorbent and preparation method and application thereof
An indole blue light and absorber technology, applied in the directions of organic chemistry, coating, etc., can solve the problems of difficult synthesis, expensive raw materials, insufficient light transmittance, etc., and achieves the effects of wide source of raw materials, simple synthesis method and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] It also provides a preparation method of poly R-indole blue light absorber, and obtains poly R-indole blue light absorber from indole monomer through free radical oxidation method;
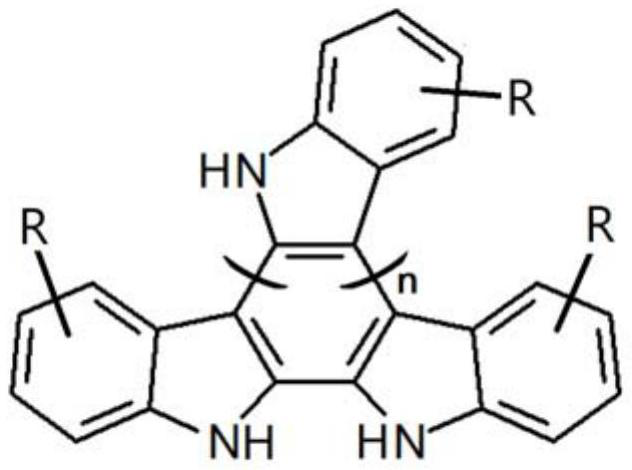
[0042] The indole monomer has a structure represented by the following general formula, also known as R-indole:
[0043]
[0044] Among them, R represents the substituent connected to the benzene ring, and the substituent connected to a benzene ring is one or two, and R is selected from -CH 3 、-C 2 h 5 、-C(CH 3 ) 3 , -CN, -F, -Cl, -Br, -I, -COOH, -OH, -CH 2 OH, -C 2 h 5 OH, -CH 2 COOH, -NH 2 , -CHO, -NO 2 、-COOCH 3 、-COOC 2 h 5 、-B(OH) 2 、-COCH 3 、-CH 2 COOCH 3 、-CH 2 COOC 2 h 5 , -OOCCH 3 , -OOCC 2 h 5 、-OCH 3 、-OC 2 h 5 、-CF 3 , -0C 6 h 5 or -OCH 2 C 6 h 5 ;
[0045] The free radical oxidation method is selected from oxidant oxidation method or ultraviolet oxidation method;
[0046] The steps of the oxidant oxidation method include: mixing indole monome...
Embodiment 1
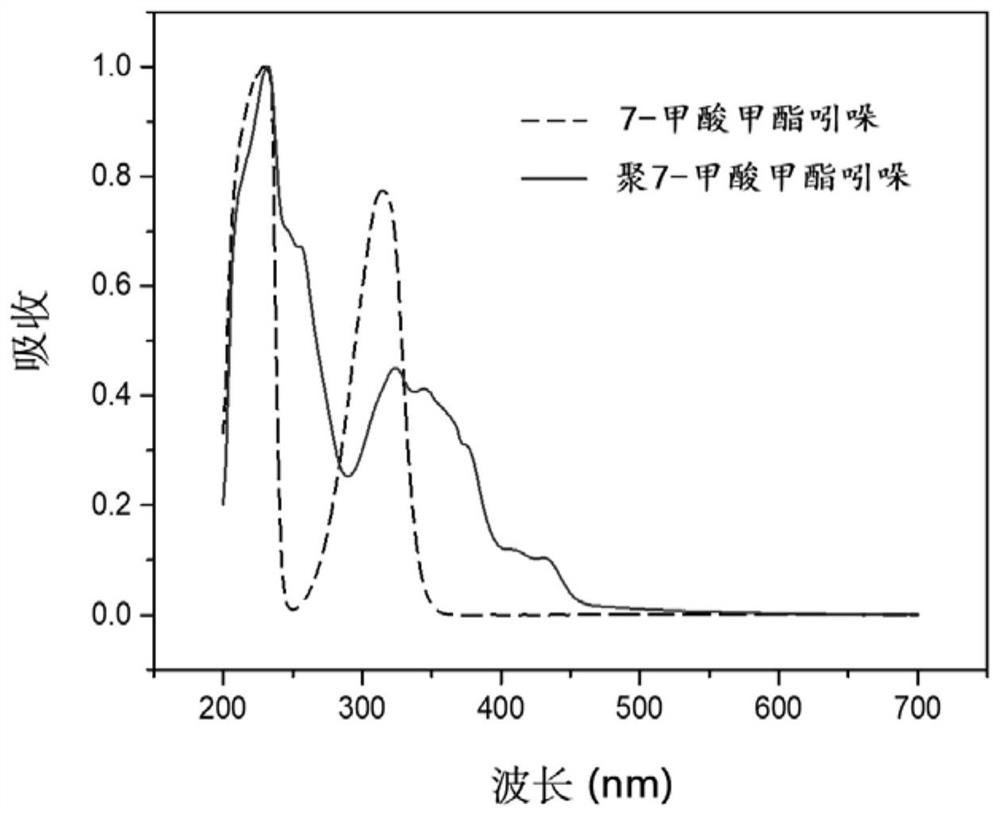
[0056] 1 part of methyl indole 7-formate, 3 parts of anhydrous ferric chloride, and 100 parts of chloroform were mixed uniformly in the reactor, stirred and reacted at room temperature for 48 hours, after filtering out impurities, the solvent was evaporated to obtain the crude product poly-7-formic acid Methyl indole, and then purified by acetone / water dissolution precipitation to obtain polymethyl indole 7-formate.
[0057] Indole 7-methylformate and polymethylindole 7-formate were prepared into ethanol solutions with a concentration of 50ppm respectively, and carried out UV-visible light spectrum analysis, the results are shown in the attached figure 1 .
[0058] Add polymethyl 7-carboxylate indole to the resin monomer of eyeglasses, the addition amount is 0.1wt%, and then solidify into a 1mm thick sheet, test its absorption effect on mobile phone screen light, record blue light, yellow light and red light The proportion of light absorbed.
Embodiment 2
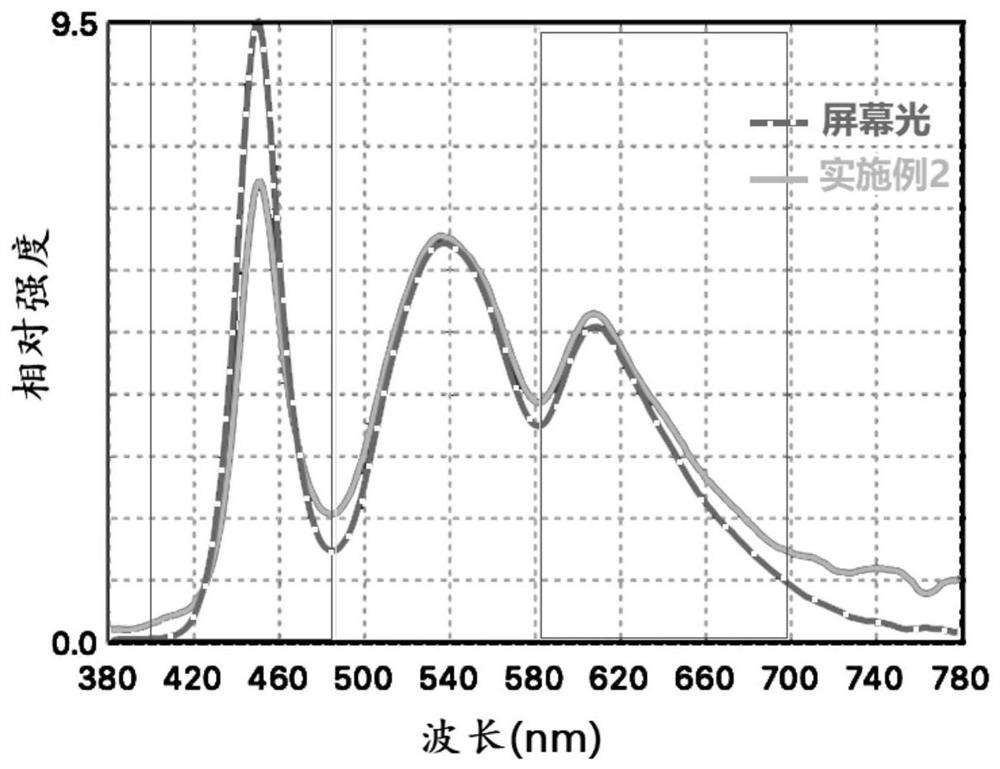
[0060] 1 part of 5-cyanylindole and 2 parts of sodium persulfate were mixed uniformly in a mortar, placed at 50°C for 24 hours, and then purified by acetone / water dissolution precipitation method to obtain poly-5-cyanylindole.
[0061] Add polymethyl-5-formate indole to the resin monomer of the eyeglass lens, the addition amount is 0.1wt%, and then solidify into a 1mm thick sheet, and test its absorption effect on the light of the mobile phone screen (attached figure 2 ), record the absorption ratio of blue light, yellow light and red light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap