Asialoglycoprotein receptor 1 (ASGR1) mutant gene and application thereof to preparation of mammal liver injury sensitive model
A mutated gene, mammalian technology, applied in the field of genetic engineering, can solve the problems of restricted development and low survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of ASGR1 gene knockout piglet
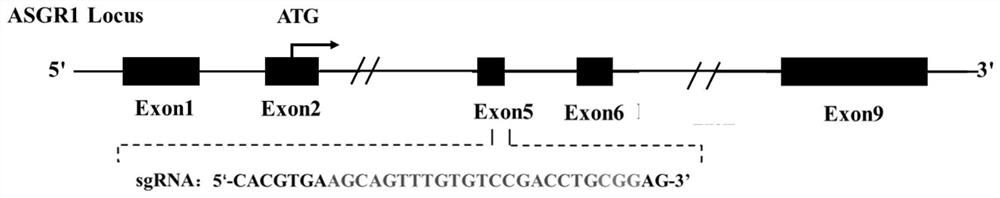
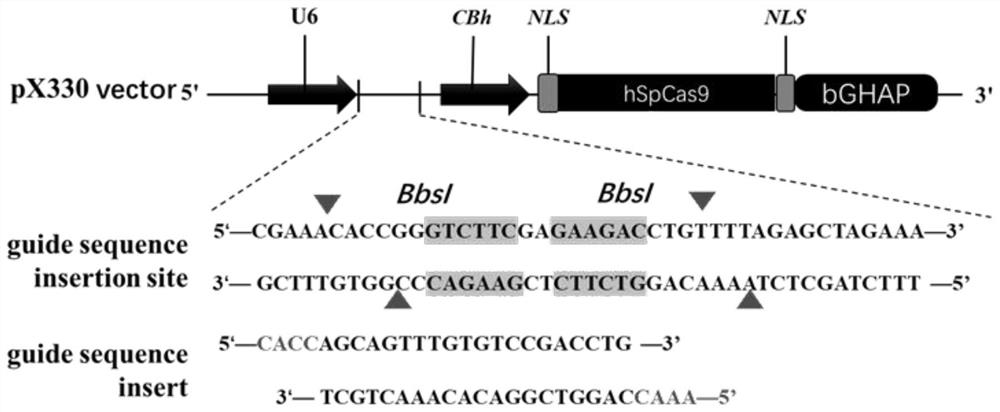
[0040] According to the pig (Sus scrofa) asialoglycoprotein receptor 1 (asialoglycoprotein receptor, ASGR1) gene sequence (Gene ID: NC_010454.4), using single-stranded guide RNA (single guide RNA, sgRNA) online design website (http: / / crispr.mit.edu / ) in its 6th exon ( figure 1 ) designed and synthesized sgRNA (sgRNA:agcagtttgtgtccgacctgcgg, SEQ ID NO:1) that specifically recognizes the target sequence DNA. After the synthesized sgRNA oligonucleotides were annealed (94°C, 10min; 37°C, 10min), they were ligated into the PX330 expression vector recovered by digestion with BbsI to construct the sgRNA expression vector ( figure 2 ). After the constructed expression vector was sequenced to verify that the connection was correct, the plasmid was extracted for cell transfection.
[0041] The validated sgRNA expression vector and Enhanced Green Fluorescent Protein (EGFP) plasmid were co-electrotransfected into Ba...
Embodiment 2
[0050] Example 2, Sensitivity of ASGR1 gene knockout piglets to liver injury
[0051] The obtained ASGR1 knockout pigs were induced by two ways of liver injury.
[0052] 1. Experimental animals
[0053] Experimental group: Offspring of ASGR1 knockout pigs (individuals produced by breeding ASGR1 knockout pigs with WT sows).
[0054] Control group: wild-type pigs (wide type, WT) without gene editing were used as controls.
[0055] The animals in the two groups were all 6 months old, gender, feeding and environment were the same.
[0056] 2. Test method:
[0057] Liver injury induction method: (1) feed pigs with 50% alcohol as a drinking water substitute for 2 months to simulate alcoholic liver injury caused by human drinking; (2) intraperitoneal injection of carbon tetrachloride for 15 days to simulate human drug-induced liver injury liver damage.
[0058] Blood test for liver injury: blood was collected before induction, and then blood biochemical indicators were tested, i...
Embodiment 3
[0069] Example 3, ASGR1 knockout piglets can be passed down normally.
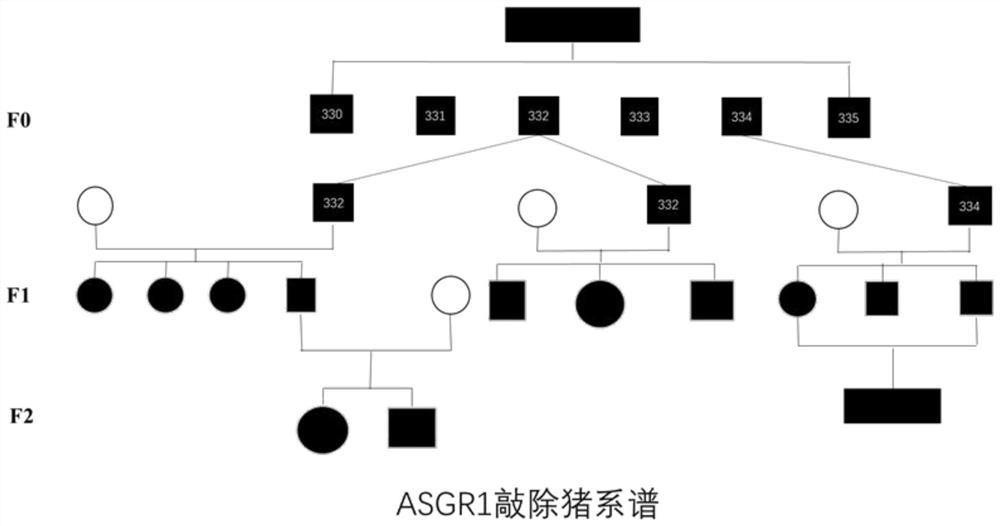
[0070] Taking ASGR1 knockout pigs as an example, 6 primary individuals were obtained through gene editing. Taking ASGR1 knockout pigs as an example, we obtained 6 primary individuals through gene editing. Select ASGR1 No. 332 and No. 334 in the original generation - / - Pigs were mated with wild-type sows, and 10 F1 ASGR1 pigs were obtained + / - pigs, to establish the pedigree of ASGR1 knockout pigs ( Figure 5 ), which has now been bred to the third generation. It shows that the present invention can prepare a population of gene-edited animals with normal breeding and stable inheritance by editing the ASGR1 gene sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com