CD3 negative cell populations expressing chemokine receptors and cell adhesion molecules and uses and methods of making same
A cell population and cell technology, applied in chemical instruments and methods, biochemical equipment and methods, biological preparations for removing undesirable cells, etc., can solve the lack of chemokine receptors, CAR-T cells do not reach solid tumors, tumors Problems such as decreased cell chemotaxis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] [Preparation method of NK-like cell population]
[0099] The present invention provides a method for preparing CCR5-positive, CCR6-positive, CXCR3-positive and CD3-negative cell populations, including the steps of preparing primary mononuclear cell populations; removing CD3-positive cells from primary mononuclear cell populations; A step in which any one selected from the group consisting of monocytes and B cells is removed from the mononuclear cell population; after removing the CD3 positive cells and any one selected from the group consisting of monocytes and B cells A step in which the remaining cell population is cultured in medium comprising IL-2.
[0100] (primary mononuclear cell population)
[0101] In the preparation method of the present invention, the primary mononuclear cell population can be obtained through the step of isolating mononuclear cells from blood cells collected from a subject. Blood cells may be collected from any one selected from the group ...
Embodiment 1
[0145]
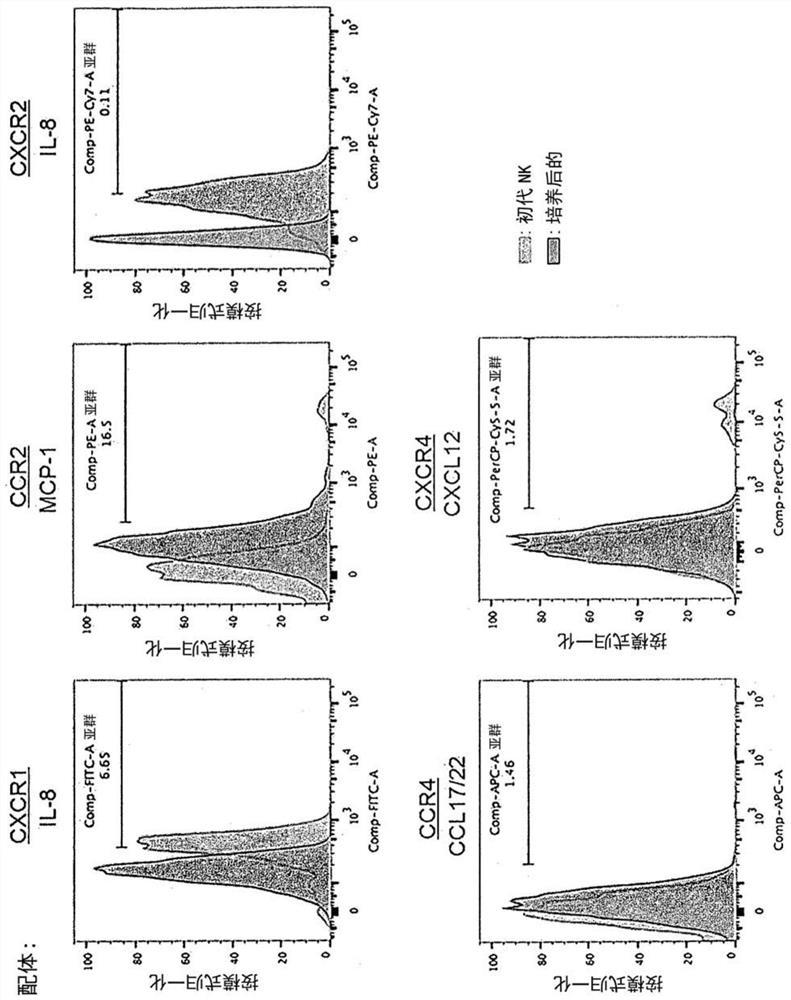
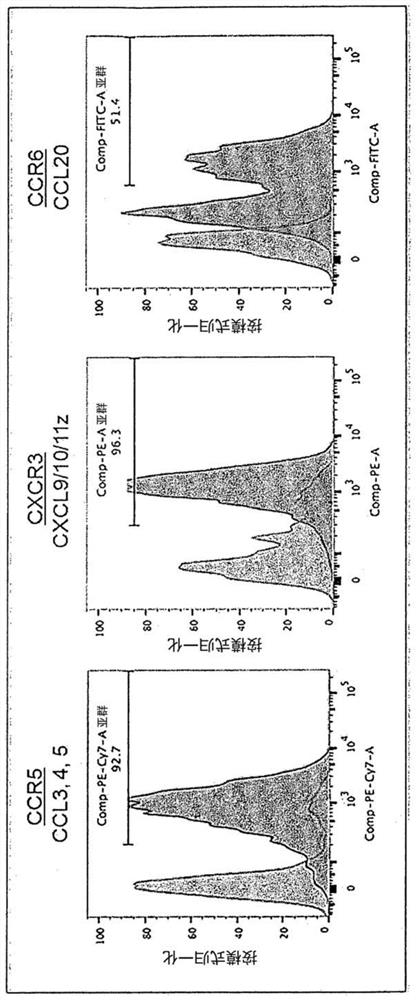
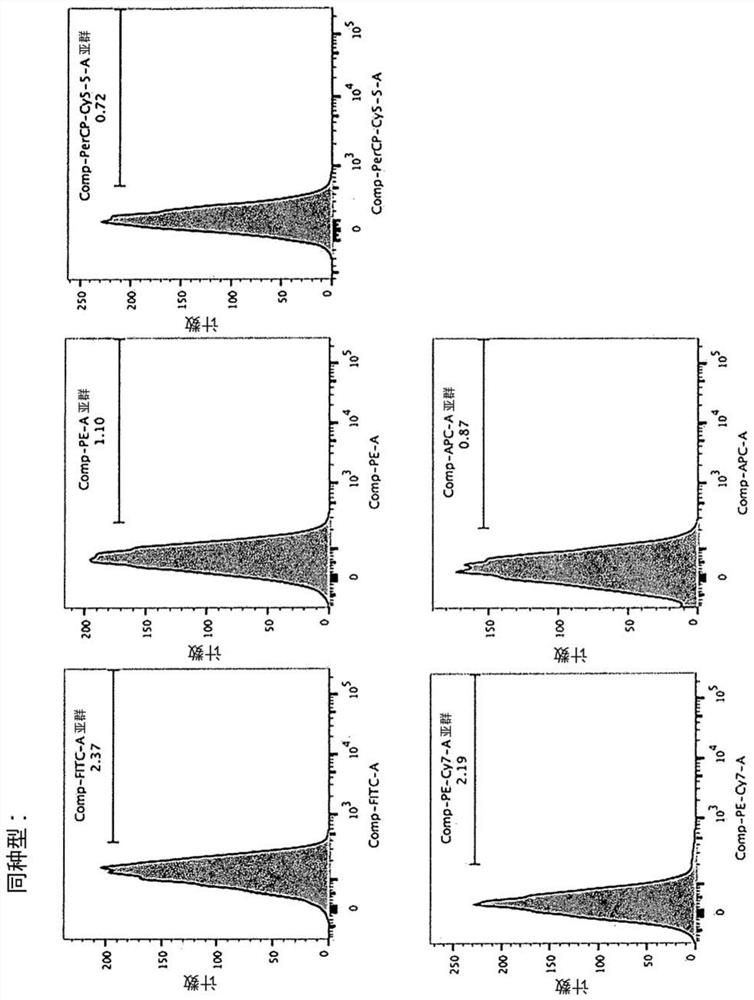
[0146] Peripheral blood of healthy volunteers was separated by density centrifugation to obtain peripheral blood mononuclear cells (hereinafter, PBMC). to each 1 x 10 7 Cells Using 5 μL of CliniMACS CD3 (Miltenyi Biotech, Cat. No. 130-017-601), CD3-positive cells were removed from PBMCs (cells recovered here were referred to as "primary NK"), and the remaining cells were cultured. In culture, the cells were divided into 5×10 5 The density of cells / mL was suspended in KBM-501 (Kohjin-bio, added with 5% AB serum), and cultured for 14 days using 6-well plates (Thermo Fisher Scientific, 140675) or T-75 flasks (Thermo Fisher Scientific, 156499) Medium was added on day 9). The cells of this cell population are referred to as "14-day cultured cells" and "cells of the present invention".
[0147] For the analysis of chemokine receptors, the cells of the present invention after 14 days of culture and "primary NK" of cells recovered before culture were used as a control. ...
Embodiment 2
[0160]
[0161] To analyze the content of CD3-positive cells and CD19-positive cells, cells of the present invention cultured for 14 days were used in the same manner as in Example 1, and PBMC were used as controls. The antibodies used for fluorescent labeling were PE-labeled anti-human CD3 antibody (Biolegend, 300408) and PerCP-Cy 5.5-labeled anti-human CD19 antibody (Biolegend, 302230). Fluorescent labeling was carried out in the same manner as in Example 1.
[0162] show the result in figure 2 . The cells of the present invention cultured for 14 days contained 0.025% of CD3 positive cells and 0.28% of CD19 positive cells. On the other hand, 64.7% of CD3-positive cells and 8.57% of CD19-positive cells were contained in PBMCs that were not cultured.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com