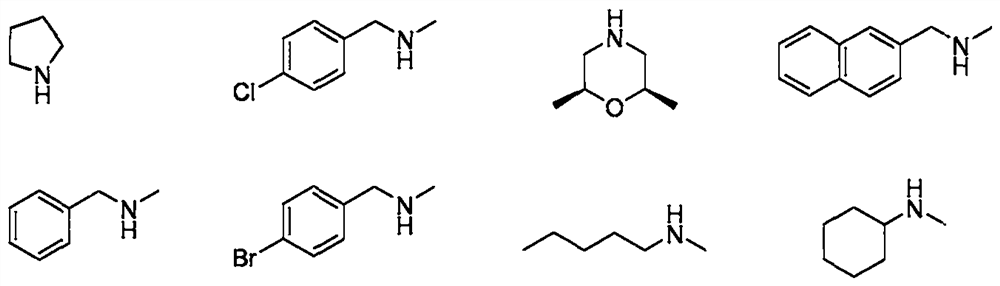
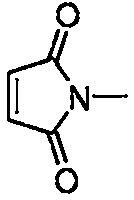
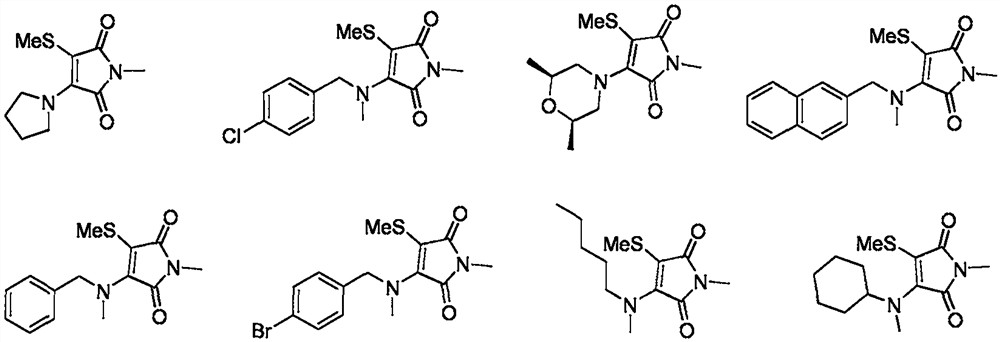
Preparation method of 3-amino-4-methylmercapto maleimide compound
A technology of methylmercaptomaleimide and methylmaleimide, which is applied in the field of organic compound synthesis, can solve the problems of high price, environmental pollution of thiophenol, lack of simplicity and high efficiency, and achieves convenient post-processing and high yield. and the effect of high purity and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of N-methyl-3-methylmercapto-4-tetrahydropyrrolylmaleimide
[0031]
[0032] At room temperature, methylmercapto Buente salt (0.6mmol, 3equiv), tetrahydropyrrole (0.6mmol, 3equiv), N-methylmaleimide (0.2mmol, 1equiv), cuprous chloride (0.04 mmol, 0.2 equiv) and 2 mL of toluene were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 120 ° C for 24 h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), the product was a yellow liquid, The yield is 95%, and the product weight is 43mg.
[0033] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0034] 1 H NMR (500MHz, CDCl 3 ): δ4.01(t, J=6.75Hz, 4H), 3.00(s, 3H), 2.23(s, 3H), 1.95(dt, J=6.30, 3.75Hz, 4H);
[0035] The data of th...
Embodiment 2
[0040] Synthesis of N-methyl-3-(4-chlorobenzylmethylamino)-4-methylmercaptomaleimide
[0041]
[0042]At room temperature, methylmercapto Buendt's salt (0.6mmol, 3equiv), N-(4-chlorobenzyl)-N-methylamine (0.6mmol, 3equiv), N-methylmaleimide (0.2 mmol, 1 equiv), cuprous chloride (0.04 mmol, 0.2 equiv) and 2 mL of toluene were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 120 ° C for 24 h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), and the product was a yellow solid. The melting point is 73-74° C., the yield is 77%, and the product weight is 48 mg.
[0043] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0044] 1 H NMR (500MHz, CDCl 3 ): δ7.33(d, J=8.30Hz, 2H), 7.17(d, J=8.15Hz,...
Embodiment 3
[0050] Synthesis of N-methyl-3-(2S,6R-2,6-dimethylmorpholinyl)-4-methylmercaptomaleimide
[0051]
[0052] At room temperature, methylmercapto Buendt salt (0.6mmol, 3equiv), cis-2,6-dimethylmorpholine (0.6mmol, 3equiv), N-methylmaleimide (0.2mmol, 1equiv), cuprous chloride (0.04mmol, 0.2equiv) and 2mL of toluene were added to the reaction tube, then filled with oxygen, and replaced three times, and stirred at a reaction temperature of 120°C for 24h. The reaction mixture was cooled, then diluted with ethyl acetate, filtered to a heart bottle, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether = 9: 1), the product was a yellow liquid, Yield 85%, product weight 46mg.
[0053] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0054] 1 H NMR (500MHz, CDCl 3 ): δ5.02(dd, J=11.35, 2.00Hz, 2H), 3.75-3.69(m, 2H), 2.98(s, 3H), 2.78(dd, J=11.35, 10.35Hz, 2H), 2.28( s, 3...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap