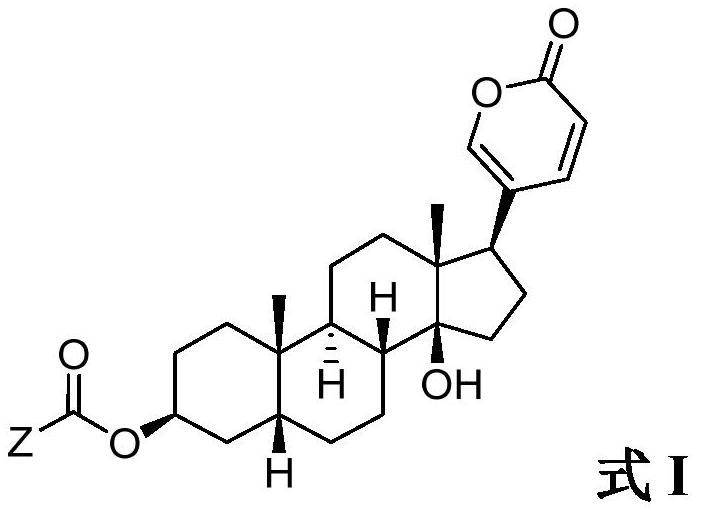
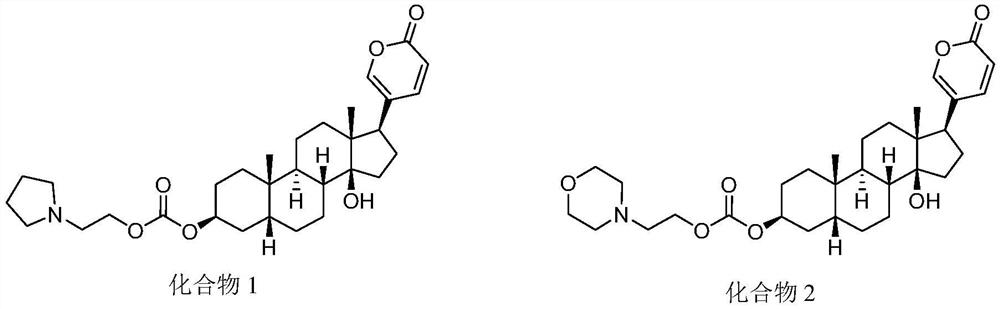
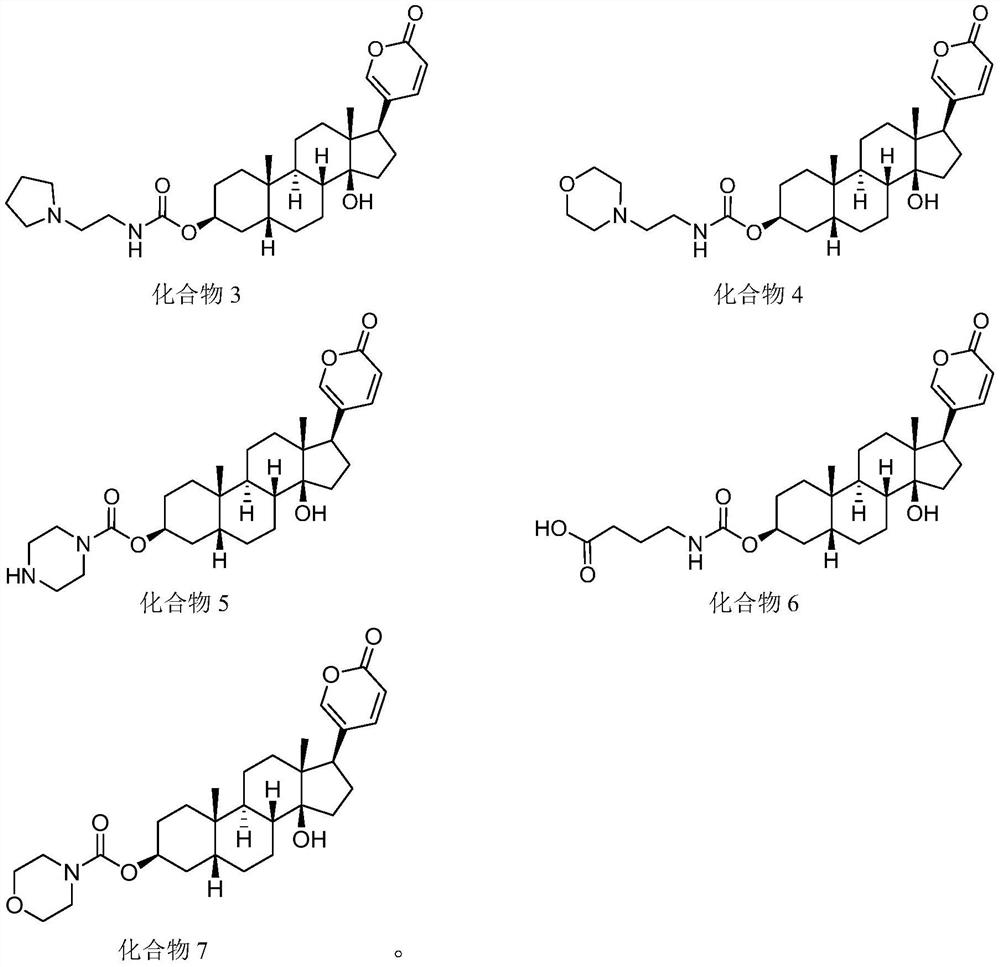
Methods of treating cancer
A cancer, optional technology, applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of unmet medical needs of patients with advanced cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] Example 1. Phase I Study of Exemplary Compounds
[0224] A Phase I study of an exemplary compound of Formula I (such as any one of the compounds listed in Table 1 ) or a pharmaceutically acceptable salt thereof is conducted in locally advanced or metastatic solid tumors.
[0225] The primary objective of the study is to evaluate patients with locally advanced or metastatic solid tumors by intravenous (IV) infusion 3 times a week (Day 1, Day 2, and Day 3 for three weeks, followed by a week off ) safety and tolerability of administered exemplary compounds of formula I, and determining the maximum tolerated dose (MTD) of exemplary compounds of formula I. If the MTD is not reached, the optimal biological dose (OBD) is determined after the Safety Review Committee (SRC) and the investigator discuss the optimal balance of toxicity, pharmacokinetics (PK), pharmacodynamics (PD), and clinical response signals ).
[0226] A secondary objective of this study was to characterize t...
Embodiment 2
[0236] Example 2. Continuous Administration of Exemplary Compounds
[0237] In this proposed study, a similar clinical trial was performed with the exemplary compound of formula I discussed in Example 1, except that the compound was administered continuously for 24 hours on days 1-14, followed by a one week rest. The safety and efficacy of this compound were evaluated as discussed in Example 1.
Embodiment 3
[0238] Example 3. Clinical Trials of Exemplary Compounds
[0239] This example provides results from clinical trials investigating the efficacy of exemplary compounds of Formula I, or pharmaceutically acceptable salts thereof, against various tumor types. Exemplary compounds of Formula I were administered to patients via 2-hour intravenous (IV) infusion once a week for three consecutive weeks. The treatment cycle was defined as 28 days, consisting of 21 days of treatment and 7 days of rest. The number of treatment cycles completed ranged from 1 to 4. Dosages of exemplary compounds of formula I range from 0.08 to 1.0 mg / m 2 . Patients in the study included both men and women. The tumor types studied in this clinical study included colorectal cancer, breast cancer, liver cancer, gastric cancer and non-small cell lung cancer. Efficacy assessments were determined at the last patient visit of the study. The results are shown in Table 2 below.
[0240] Table 2
[0241]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com