Preparation method of 2-amino-4, 6-dichloro-5-formamidopyrimidine
A technology of formamide and pyrimidine, which is applied in the field of compound preparation, can solve the problems of difficult removal of phosphorus-containing wastewater, difficult large-scale production operation, and difficult discharge of qualified standards, and achieves the effects of industrial production, improved safety, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
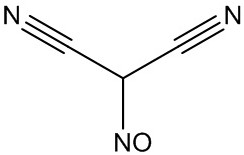
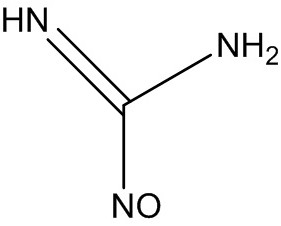
[0075] Synthesis of Nitrosomalononitrile (I), Nitrosoformamidine (III)
[0076] Add 165.0g (2.5mol) of malononitrile and 825g of 25% hydrochloric acid into a 2000mL four-necked reaction flask, start stirring, cool down to 0°C, add 593g of 35% sodium nitrite solution dropwise, and react at room temperature for 2 hours (detection The raw material was HPLC<0.3%), cooled to 5°C, filtered and dried by suction to obtain 235.1 g of a solid, the purity of which was measured by HPLC was 99.5%, and the yield was 99.0% (calculated as malononitrile).
[0077] Add 201.3g (2.5mol) of formamidine hydrochloride and 1005g of 25% hydrochloric acid into a 2000mL four-necked reaction flask, start stirring, cool down to 0°C, add 593g of 35% sodium nitrite solution dropwise, and react at room temperature for 2 hours (detection Raw material HPLC<0.3%), cooled to 5°C, suction filtered and dried to obtain 181.0 g of solid, the purity measured by HPLC was 99.5%, and the yield was 99.2% (calculated as f...
Embodiment 2
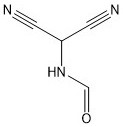
[0079] Synthesis of formamidomalononitrile (II)
[0080] Add 230g (2.42mol) of nitrosomalononitrile, 690.0g formic acid, and 95.0g iron powder into a 1000mL four-necked reaction flask, stir, heat up to 100°C and reflux for 4 hours (HPLC for detection of raw materials<0.5%), cool down to Filtrate at 50°C, apply the filter cake (KF detection is below 0.5%), distill formic acid from the filtrate under reduced pressure until the solid precipitates, then slowly cool down to 0°C, filter and dry to obtain 251.0 g of solid, the purity of which is 99.0% by HPLC. The yield was 95.2%.
Embodiment 3
[0082] Synthesis of formamidomalononitrile (II)
[0083] Add 230g (2.42mol) of nitrosomalononitrile, 690.0g formic acid, and 110.0g zinc powder into a 1000mL four-necked reaction flask, stir, heat up to 100°C and reflux for 4 hours (HPLC for detection of raw materials<0.5%), cool down to Filtrate at 50°C, apply the filter cake (KF detection is less than 0.5%), distill formic acid from the filtrate under reduced pressure until the solid precipitates, then slowly cool down to 0°C, filter and dry to obtain 252.0g of solid, the purity of which is 99.1% by HPLC. The yield was 95.5%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap