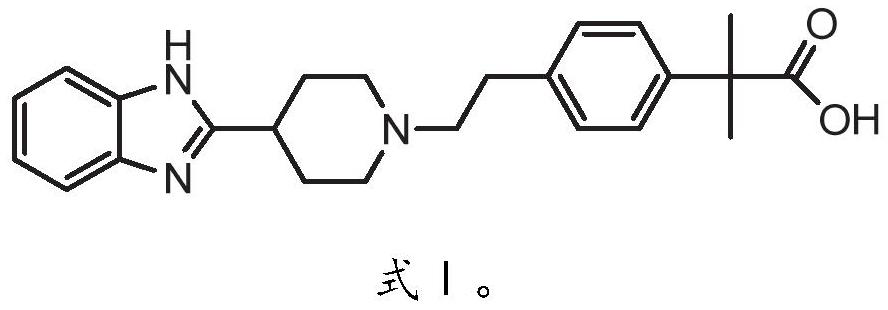
Method for preparing bilastine
A compound, ethyl technology, applied in the field of drug synthesis, can solve problems such as difficult removal of impurities, increased post-processing difficulty, and fracture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
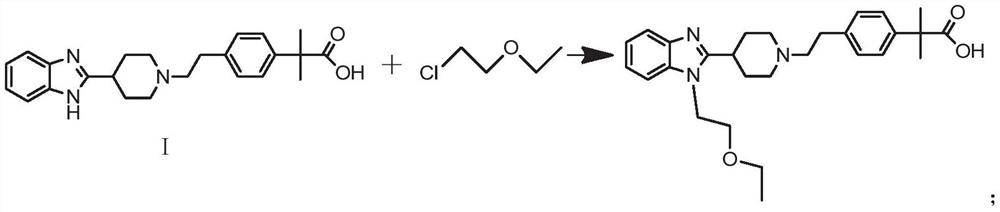
[0027] In a more preferred preparation scheme, bilastine is prepared by reacting the compound represented by formula I with 2-chloroethyl ether.
[0028] In some embodiments of the above-mentioned preferred preparation method, the preparation method of bilastine is prepared by reacting the compound shown in formula I with 2-chloroethyl ether, and the reaction is shown in the following formula:
[0029]
[0030] The preparation method is as follows:
[0031] 2-(4-{2-[4-(1H-benzimidazol-2-yl)-piperidin-1-yl]-ethyl}-phenyl)-2-methyl-propionic acid (Formula I shown compound), DMF and sodium hydride were mixed and reacted for a period of time, then 2-chloroethyl ether was added, and the heating reaction was continued. After the reaction, the pH value of the reaction system was adjusted to neutral by glacial acetic acid, and a white solid was obtained by filtration, which was the ratio Rustin.
[0032] In this series of embodiments, the 2-(4-{2-[4-(1H-benzimidazol-2-yl)-piperid...
Embodiment 1
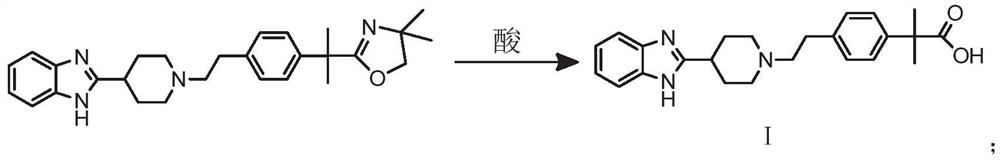
[0059] In this embodiment, a kind of 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-1-methanol Base-ethyl]-phenyl}-ethyl)-piperidin-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole, 2-(4-{2-[ 4-(1H-benzimidazol-2-yl)-piperidin-1-yl]-ethyl}-phenyl)-2-methyl-propionic acid is an intermediate bilastine preparation method, the The preparation method is shown in the following formula:
[0060]
[0061] Step 1: Preparation of 2-[1-(2-{4-[1-(4,4-Dimethyl-4,5-dihydro-oxazol-2-yl)-1-methyl-ethyl] -Phenyl}-ethyl)-piperidin-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole
[0062] 20.1g 2-piperidin-4-yl-1H-benzimidazole, 41.6g 2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl )-1-methyl-ethyl]-phenyl}-ethyl p-toluenesulfonate, 11.7g of sodium carbonate, 50ml of DMF were put into the reaction flask, and heated to 80°C for 2 hours. Add 100ml of deionized water, filter, and air-dry the filter cake at 70°C for 3 hours to obtain 35.5g of white solid. Yield 79.8%.
[0063] Step 2: Preparation of ...
Embodiment 2
[0068] The difference from Example 1 is that another method for preparing 2-(4-{2-[4-(1H-benzimidazol-2-yl)-piperidin-1-yl]-ethyl Base}-phenyl)-2-methyl-propionic acid method:
[0069] 40.0g 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-1-methyl-ethyl]- Phenyl}-ethyl)-piperidin-4-yl]-1-(2-ethoxyl-ethyl)-1H-benzimidazole, 60ml ethanol, 10ml concentrated hydrochloric acid are put into the reaction flask and heated to The reaction was refluxed for 5 hours. After the reaction, the pH value was adjusted to 7 with 4 mol / L sodium hydroxide, filtered, and the filter cake was washed with water, and dried at 70° C. for 3 hours to obtain 31.8 g of a white solid. Yield 90.3%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap