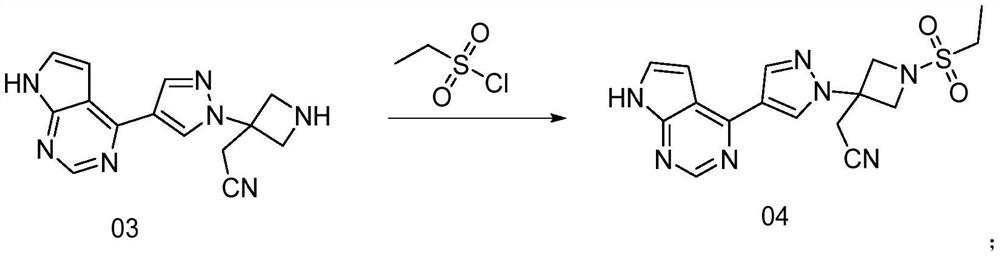
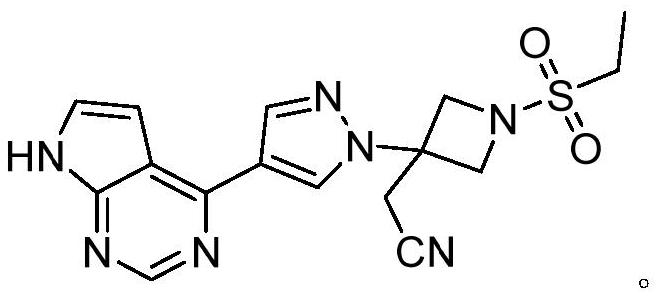
Preparation method of azetidine compound
A compound and catalyst technology, applied in the field of preparation of azetidine compounds, can solve the problems of cumbersome post-processing process, unfavorable industrialization, and many reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
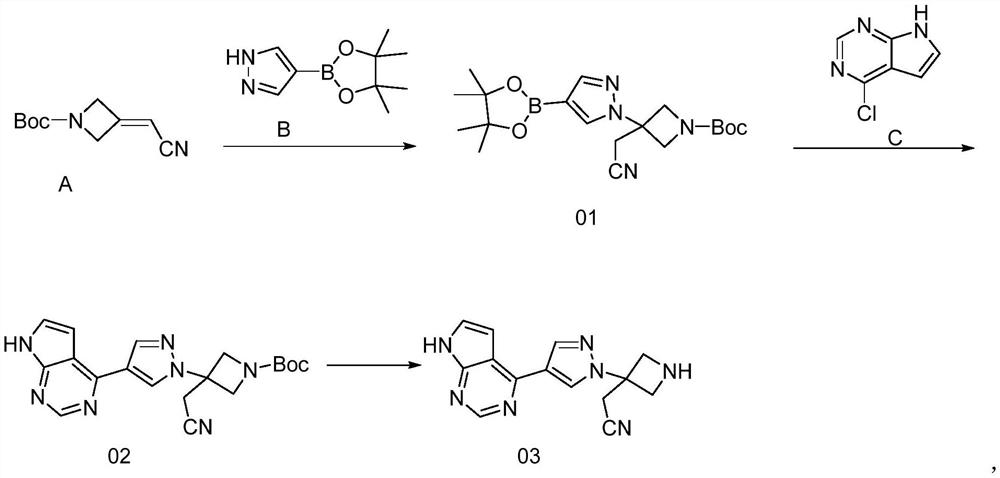
[0060]Example 1: Preparation of Compound A
[0061]Add toluene (400mL) and potassium tert-butoxide (68.17g, 608mmol) into the reaction flask; control to 0±5℃, add cyanomethyl diethyl phosphate solution (106.58g, 602mmol) dropwise to the system. After the addition, control the temperature to 0±5℃, keep stirring for 0.5h; slowly add N-Boc cyclobutanone solution (100g, 584mmol) dropwise, after the addition, control the temperature at 0±5℃ to react for 2-3h; add to the system It was quenched with water (500 mL), heated to 25±5°C, stirred for 15 min, allowed to stand for layering, and separated; the organic phase was used directly in the next step without treatment; purity by HPLC: 98.7%.
Embodiment 2
[0062]Example 2: Preparation of Compound 01
[0063]At 25°C, add 1,8-diazabicyclo[5.4.0]undec-7-ene (that is, the reaction flask containing compound A (the yield is calculated as 100%) obtained in Example 1) DBU, 88.95g, 585mmol), compound B (115.61g, 596mmol) was heated to 80±5°C and reacted for 2h-3h; the solvent was concentrated under reduced pressure to obtain compound 01 concentrate with a purity of 99.85%, which was directly used in the next reaction.
Embodiment 3
[0064]Example 3: Preparation of Compound 02
[0065]At 25±5℃, add n-butanol (750mL), water (250mL), compound C (39.61g) to a reaction flask containing compound 01 (100g, 258mmol, the concentrate obtained in Example 2 is converted according to the purity data). , 258mmol), NaHCO3(32.45g, 386mmol), tetrakis(triphenylphosphine) palladium (1.49g, 1.29mmol); Replace nitrogen three times, warm up to 85±2℃, keep stirring for 15h-16h; after the reaction is over, the reaction solution is directly without post-treatment For the next step, the reaction solution was tested, and the purity of the product was 98.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com