Big data-oriented potential adverse drug reaction data mining system and method
A technology of adverse reactions and data mining, which is applied in the fields of medical data mining, drug reference, patient-specific data, etc., can solve problems such as inability to perform effective analysis, inability to analyze drugs, and large amount of calculation, so as to facilitate understanding and analysis, increase The effect of large sample accuracy and large amount of calculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
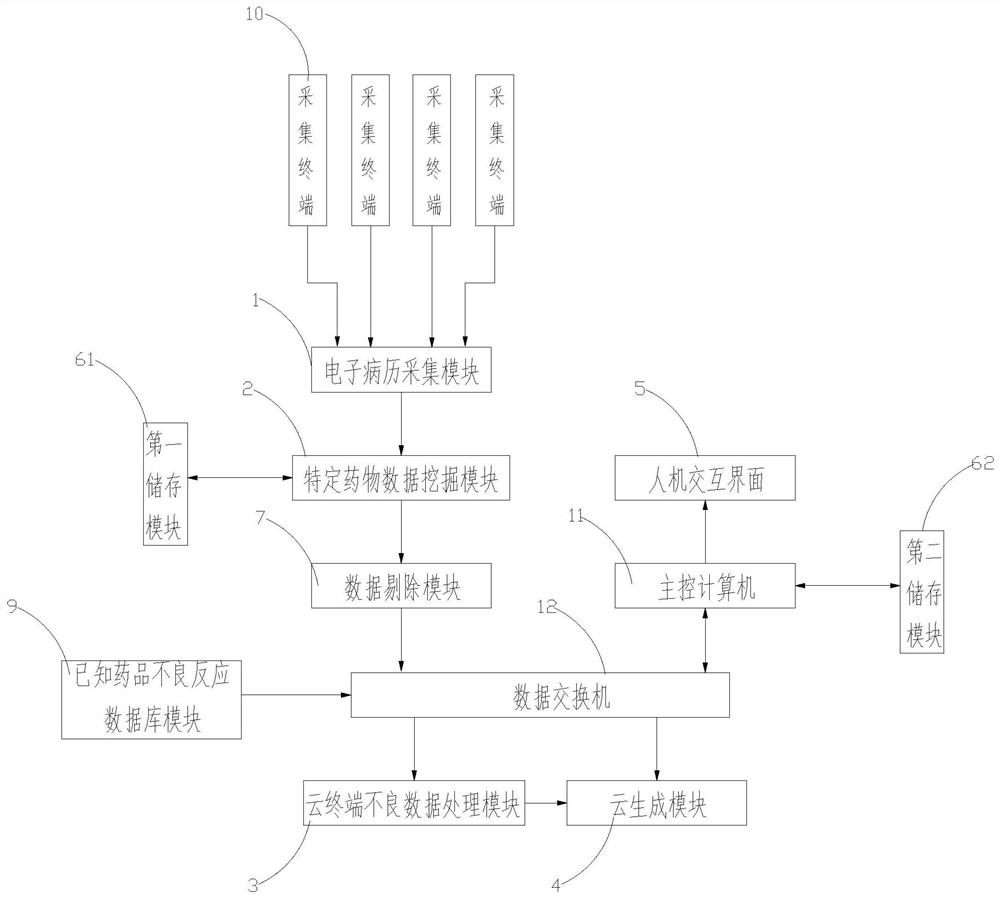
[0043] refer to figure 1 , the present invention is a big data-oriented potential adverse drug reaction data mining system, comprising.
[0044] A plurality of acquisition terminals 10. The collection terminal 10 is set in each hospital and connected with the electronic medical record system in the hospital for data communication. It is used to network the electronic medical record systems of various hospitals and collect electronic medical records.
[0045] Electronic medical record collection module 1. Connect with collection terminal 10. It is used to network the electronic medical record systems of various hospitals. Collect electronic medical records.
[0046] Drug-Specific Data Mining Module2. It is connected with the electronic medical record collection module 1. It is used to mine patients who have taken specific medicines in the electronic medical records collected by the electronic medical record collection module 1 . Get relevant patient information.
[0047]...
Embodiment 2
[0057] The present invention is a big data-oriented potential adverse drug reaction data mining method, comprising the following steps.
[0058] Step S1. The electronic medical record system of each hospital is networked.
[0059] Step S2. Capture patients who have taken specific drugs in the electronic medical record system, establish a list of drug users, and obtain relevant patient information.
[0060] Step S3. According to the list of patients captured in step S2, the adverse event data in the patient's medical record after taking the drug is extracted, and a database of related drugs and adverse reactions after using the drug is established.
[0061] Step S4. Screen related drugs and patient data in the database of adverse reactions after using the drug, and remove some useless patient data.
[0062] Step S5. Classify by feature, classify the patient data screened in step S4 by feature, classify similar adverse reactions together, generate a database table of relat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com