A fast-dissolving favipiravir pharmaceutical composition and preparation method
A favipiravir and composition technology, which is applied in the field of favipiravir pharmaceutical compositions, can solve the problems of poor production feasibility and achieve the effects of small tablet weight, improved bioavailability, and increased compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044]
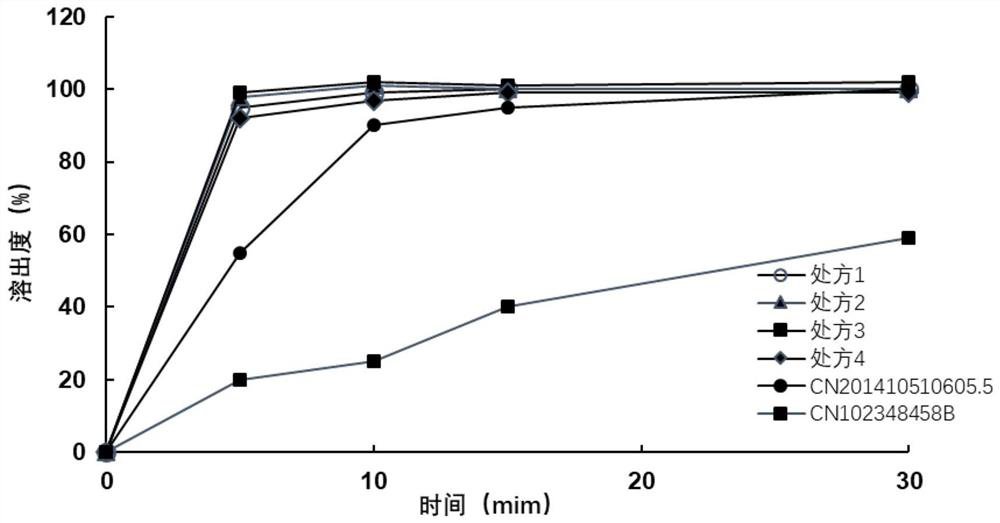
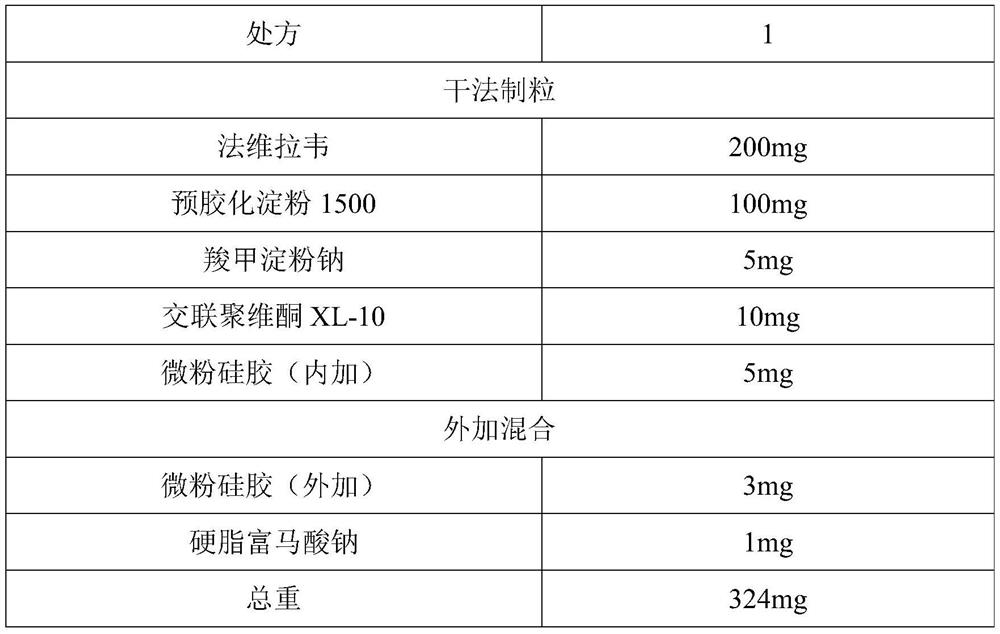
[0045] Favipiravir is jet-milled to collect materials, requiring D90≤100 microns, D50=5-30 microns, and D10=0.1-8 microns. Weigh the crude drug and auxiliary materials according to the above prescription ratio, and pass through a 40-mesh sieve respectively. Put the raw material favipiravir and the auxiliary materials pregelatinized starch 1500, sodium starch glycolate, crospovidone XL-10, and micropowder silica gel (internal addition) in a multi-directional motion mixer for mixing for 15 minutes , mix the materials evenly. Put the above materials into the dry granulator, adjust the parameters of the feeding module and the rolling module respectively: the horizontal speed is 5rpm, the vertical speed setting is 100rpm, the roller gap is 2.0mm, and the roller pressure is 10±1KN / cm 2 . Within the above range, by adjusting the horizontal rotation speed, vertical rotation speed, roller gap, and roller pressure, the thin slices with appropriate hardness can ...
Embodiment 2
[0047]
[0048]
[0049] Favipiravir is jet-milled to collect materials, requiring D90≤100 microns, D50=5-30 microns, and D10=0.1-8 microns. Weigh the crude drug and auxiliary materials according to the above prescription ratio, and pass through a 40-mesh sieve respectively. All the other operations are the same as in Example 1.
Embodiment 3
[0051]
[0052] Favipiravir is jet-milled to collect materials, requiring D90≤100 microns, D50=5-30 microns, and D10=0.1-8 microns. Weigh the crude drug and auxiliary materials according to the above prescription ratio, and pass through a 40-mesh sieve respectively. All the other operations are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com