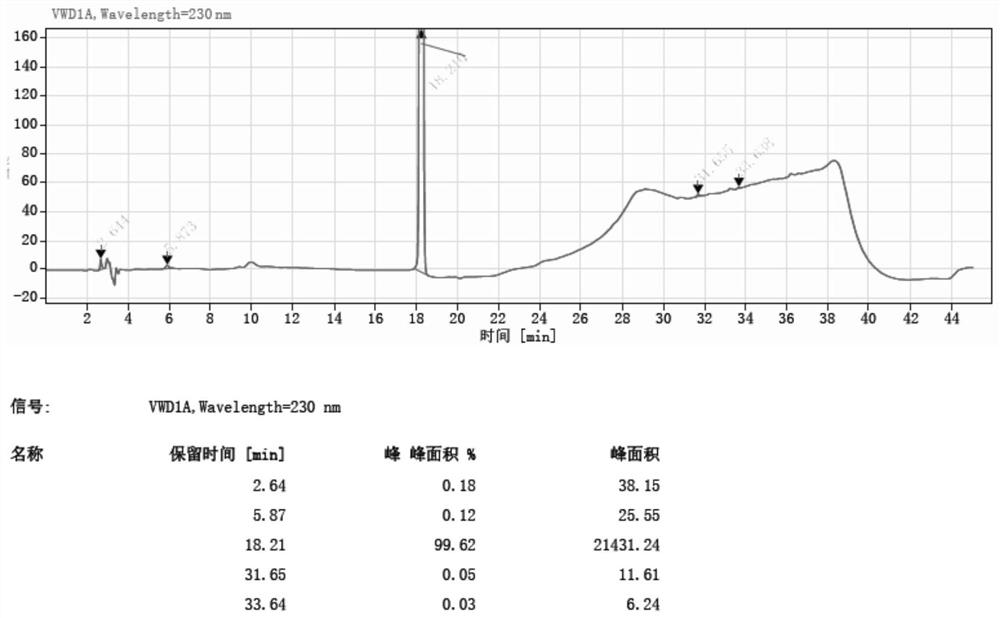
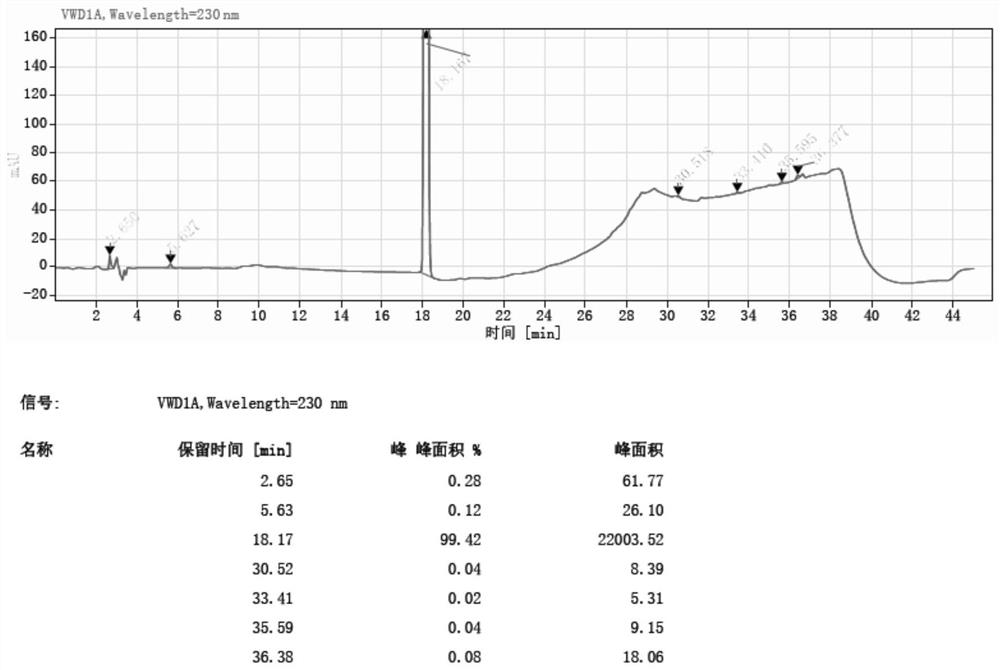
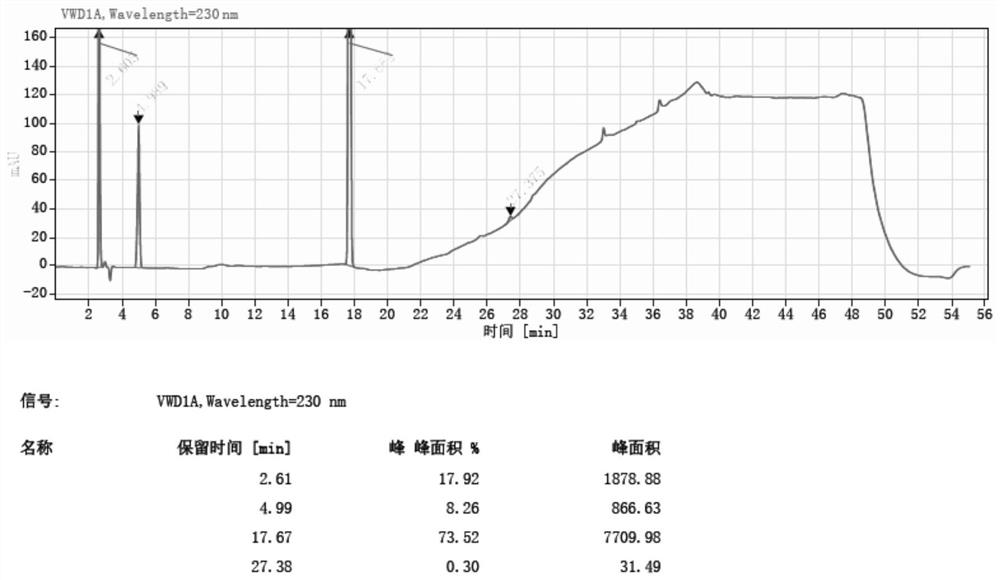
Method for synthesizing 5-nitro-2-(propylthio) pyrimidine-4, 6-diol through continuous nitration
A technology based on propylsulfide and propylthio, which is applied in the field of continuous nitration synthesis of 5-nitro-2-pyrimidine-4,6-diol, which can solve the problems of excessive local concentration, overheating, explosion, etc., and achieve Effects of avoiding the use of nitric acid, reducing emissions, and reducing safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0045] Example 1:
[0046] The microchannel reactor was washed with drinking water, and the temperature control device temperature of the reactor was set to 25 ° C, and the ozone cylinder was connected to the microchannel reactor feed port C by the stainless steel pipe. It is weighted 4,6-dihydroxy-2- (propylthio) pyrimidine hydrochloride 10 g (38.6 mmol) to 100 ml of beaker A, and 50 ml of drinking water is added to the beaker A, stirred and dissolved, and spare. 30 ml of 10% sodium hydroxide solution, poured into the beaker D, and the magnetic stirrer was placed in the beaker D and placed on a magnetic stirrer, and the pipe at the discharge port D was attached to the beaker D, spare.
[0047] The feed pump A is opened, and the flow rate of the feed pump A is set to 4 ml / min, and the material in the beaker A is input into the microchannel reactor. After inputting the reactor 8 seconds in the beaker A, the feed pump B is turned on, and the flow rate of the feed pump B is 113 mL ...
Example Embodiment
[0057] Example 2:
[0058] The microchannel reactor was washed with drinking water, and the temperature control device temperature of the reactor was set to 35 ° C, and the ozone cylinder was connected to the microchannel reactor feed port C by stainless steel pipes. It is weighted 4,6-dihydroxy-2- (propylthio) pyrimidine hydrochloride 10 g (38.6 mmol) to 100 ml of beaker A, and 50 ml of drinking water is added to the beaker A, stirred and dissolved, and spare. 30 ml of 10% sodium hydroxide solution, poured into the beaker D, and the magnetic stirrer was placed in the beaker D and placed on a magnetic stirrer, and the pipe at the discharge port D was attached to the beaker D, spare.
[0059] The transfer pump A is opened, and the flow rate of the feed pump A is opened to 8 ml / min, and the material in the beaker A is input in the microchannel reactor. After inputting the reactor 8 seconds in the beaker A, the feed pump B is turned on, and the flow rate of the feed pump B is 226 m...
Example Embodiment
[0061] Example 3:
[0062] The microchannel reactor was cleaned with a drinking water, and the temperature of the reactor was set to 15 ° C, and the ozone cylinder was attached to the microchannel reactor feed port C by stainless steel pipes. It is weighted 4,6-dihydroxy-2- (propylthio) pyrimidine hydrochloride 10 g (38.6 mmol) to 100 ml of beaker A, and 50 ml of drinking water is added to the beaker A, stirred and dissolved, and spare. 30 ml of 10% sodium hydroxide solution, poured into the beaker D, and the magnetic stirrer was placed in the beaker D and placed on a magnetic stirrer, and the pipe at the discharge port D was attached to the beaker D, spare.
[0063] The transfer pump A is opened, and the flow rate of the feed pump A is set to 2 ml / min, and the material in the beaker A is input to the microchannel reactor. After the material in the beaker A, after 8 seconds, the feed pump is turned on, and the flow rate of the feed pump B is 59 ml / min, open the nitrogen dioxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com