Preparation method of isoquinolinone compound
A compound and methylation technology, which is applied in the field of preparation of isoquinolinone compounds, can solve the problems of high equipment requirements, unfavorable industrial production, long reaction route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
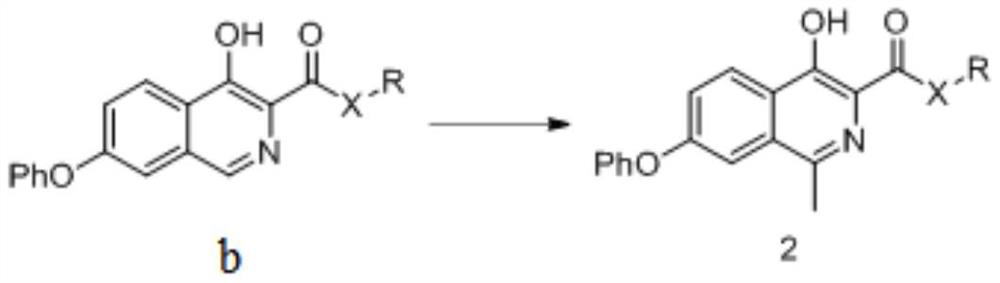
[0199] The preparation method of formula 2 compound
[0200] The present invention provides a kind of preparation method of the compound of formula 2 described in the present invention, described method comprises steps:
[0201] (i) the compound of formula b is reacted with a methylating reagent to obtain the compound of formula 2;
[0202]
[0203] In a preferred example of the present invention, in the step (i), the methylation reagent includes (but not limited to): tert-butanol peroxide, acetaldehyde and peroxide mixture, or a combination thereof.
[0204] In another preferred example, the peroxide includes (but not limited to): hydrogen peroxide, tert-butyl alcohol peroxide, di-tert-butyl peroxide, or a combination thereof.
[0205] In another preferred example, the peroxide is hydrogen peroxide.
[0206] In another preferred example, in the mixture of acetaldehyde and peroxide, the molar ratio of acetaldehyde to peroxide is 1-20:1, preferably 1-15:1, More preferably...
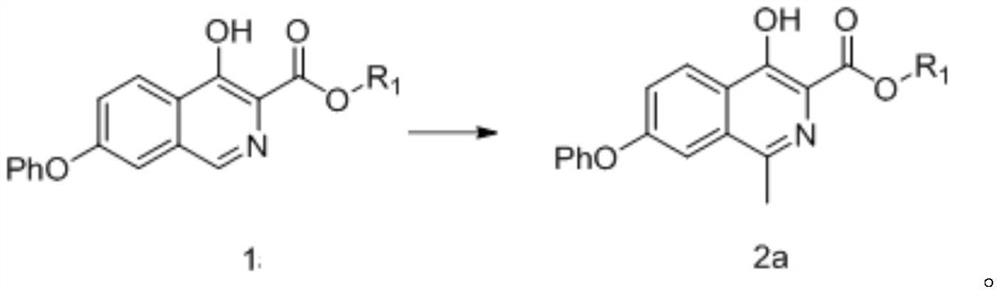
Embodiment 1
[0298]
[0299] 4-Hydroxy-7-phenoxyisoquinoline-3-formic acid methyl ester (compound of formula 1a-1) (10g, 33.9mmol) and acetic acid (100ml) were mixed, slowly added dropwise the sulfuric acid of ferrous sulfate (68mmol) Aqueous solution (dissolve ferrous sulfate in 100ml of water, add 50ml of concentrated sulfuric acid dropwise under ice bath to obtain sulfuric acid aqueous solution of ferrous sulfate), then cool down to -25°C and add acetaldehyde (340mmol), control temperature -25°C~-15°C , add hydrogen peroxide (68mmol) dropwise, then control the temperature at -15°C to -5°C for 3h, TLC plate detection reaction is complete, add sodium thiosulfate aqueous solution to stir, add dichloromethane to extract, concentrate the organic phase to obtain 4-hydroxy- 9.5 g of methyl 1-methyl-7-phenoxyisoquinoline-3-carboxylate (compound of formula 2a-1), yield 90.7%, purity 98.1% as determined by HPLC.
[0300] 1 HNMR(400MHz,DMSO)δ12.10(s,1H),8.30(d,J=9.0Hz,1H),7.62(d,J=2.3Hz,1H),7....
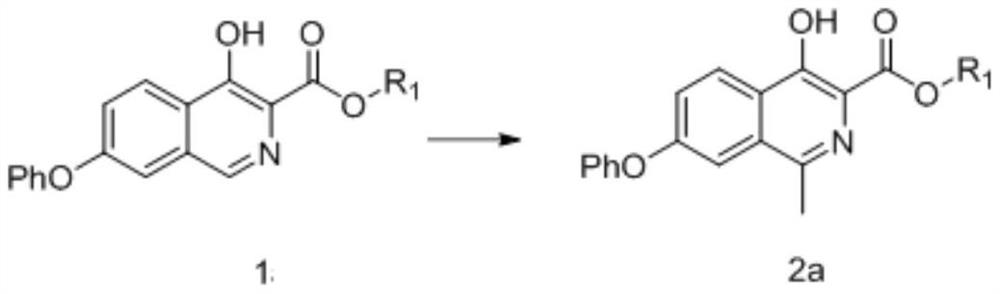
Embodiment 2
[0303]
[0304]Mix 4-hydroxy-7-phenoxyisoquinoline-3-carboxylic acid phenyl ester (compound of formula 1a-2) (12.1g, 33.9mmol) with 121ml of acetic acid and 121ml of tetrahydrofuran, and slowly add ferrous sulfate (68mmol) dropwise Aqueous solution of sulfuric acid (dissolve ferrous sulfate in 100ml of water, add 50ml of concentrated sulfuric acid dropwise under ice bath to obtain aqueous sulfuric acid solution of ferrous sulfate), then cool down to -25°C, control temperature -25°C~-15°C and add peroxide After the tert-butanol (135.6mmol) was dropped, the temperature was controlled at -15°C to -5°C for 3 hours, and the TLC plate detected that the reaction was complete, adding aqueous sodium thiosulfate solution and stirring, adding dichloromethane for extraction, and concentrating the organic phase to obtain 4-hydroxy- 10.8 g of methyl 1-methyl-7-phenoxyisoquinoline-3-carboxylate (compound of formula 2a-2), yield 85.9%, purity 97.8% as determined by HPLC.
[0305] 1 HNMR(4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com