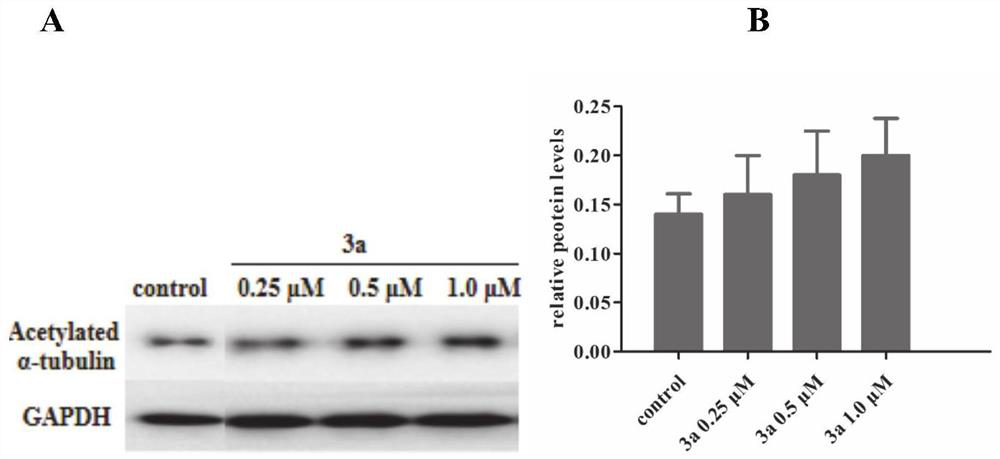
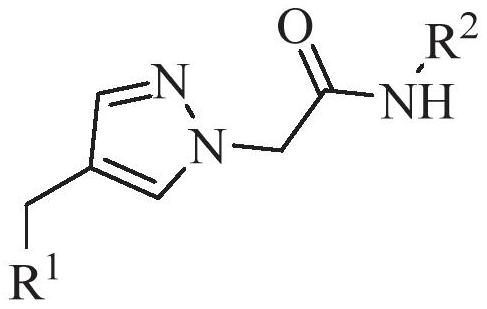
A compound for improving α-tubulin acetylation level and its preparation method and application
A compound and drug technology, applied in the field of medicine, can solve problems such as the burden on the patient's family, high cost of treatment and care, and inability to delay the progress of the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] In yet another specific embodiment of the present invention, a preparation method of the above-mentioned compound is provided, the preparation method comprising:
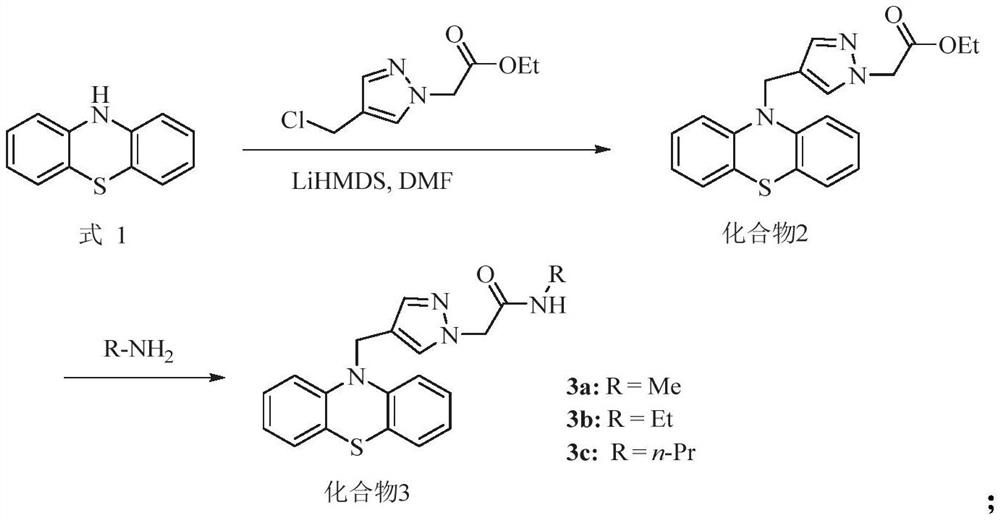
[0049] a) When the compound is compound 3, its synthetic route is as follows:
[0050]
[0051] Those skilled in the art can obtain better reaction conditions through experimentation according to the above-mentioned route. As an illustration, the present invention further provides some specific implementation modes. The synthetic method of described compound 3 comprises:
[0052] (1) Dissolve phenothiazine in N,N-dimethylformamide, under low temperature conditions, add lithium bistrimethylsilylamide for reaction, then add 4-chloromethylpyrazole-1-ethyl acetate Ester, reaction under low temperature conditions; separation and purification, to obtain compound 2, the structural formula of compound 2 is
[0053] (2) dissolving the compound 2 obtained in step (1) in the fatty amine solution, stirring and reac...
Embodiment 1
[0078] Embodiment 1: the preparation of compound 2
[0079] (1) Dissolve phenothiazine (Formula 1) (125mg, 0.61mmol) in 2mL of N,N-dimethylformamide, and drop lithium bistrimethylsilylamide (0.8mL , 0.82mmol), stirred for 30min. Afterwards, ethyl 4-chloromethylpyrazole-1-acetate (190 mg, 0.94 mmol) was dissolved in 2 mL of N,N-dimethylformamide, and then dropped into the reaction solution. Stir at -20°C for 3h. Extract with ethyl acetate, combine organic phase, wash with saturated NaCl, anhydrous NaCl 2 SO 4 Dry, filter and concentrate. Silica gel column chromatography gave ethyl 2-[4-((10H-phenothiazin-10-yl)methyl)-1H-pyrazol-1-yl]acetate (2), yellow oil, 100mg, yield rate 56%. 1 H NMR (400MHz, CDCl 3 )δ7.45(s,1H),7.25(s,1H),7.04–6.93(m,4H),6.84–6.68(m,4H),4.91(s,2H),4.76(s,2H),4.12 (q, J=7.1Hz, 2H), 1.17(t, J=6.0Hz, 3H); ESI-MSm / z: 388.06[M+Na] + .
Embodiment 2
[0080] Embodiment 2: the preparation of compound 3
[0081] (1) Dissolve ethyl 2-[4-((10H-phenothiazin-10-yl)methyl)-1H-pyrazol-1-yl]acetate (2) (50mg, 0.14mmol) in 2mL formaldehyde In aqueous amine solution, react at room temperature for 5h. Quenched with water, extracted with ethyl acetate, combined organic phases, washed with saturated NaCl, anhydrous NaCl 2 SO 4 Dry, filter and concentrate. Silica gel column chromatography gave N-methyl-2-[4-((10H-phenothiazin-10-yl)methyl)-1H-pyrazol-1-yl]amide (3a), white solid, 32mg , yield 78%. Melting point: 150–152°C; 1 H NMR (400MHz, CDCl 3 )δ7.51(s,1H),7.27(s,1H),7.04(d,J=7.6,Hz,2H),7.01–6.93(m,2H),6.82(t,J=7.5Hz,2H) ,6.68(d,J=7.9Hz,2H),6.02(s,1H),4.90(s,2H),4.64(s,2H),2.66(d,J=4.8Hz,3H); ESI-MS m / z:373.05[M+Na] + .
[0082] (2) Dissolve ethyl 2-[4-((10H-phenothiazin-10-yl)methyl)-1H-pyrazol-1-yl]acetate (2) (50mg, 0.14mmol) in 2mL ethyl alcohol In aqueous amine solution, react at room temperature for 5h. Quenched with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com