Use of lauryl diamine derivatives in preparation of drugs for treatment of breast cancer
A technology of lauryl diamine and derivatives, applied in the field of medicine, can solve the problems of uncontrollable disease and lack of breast cancer cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
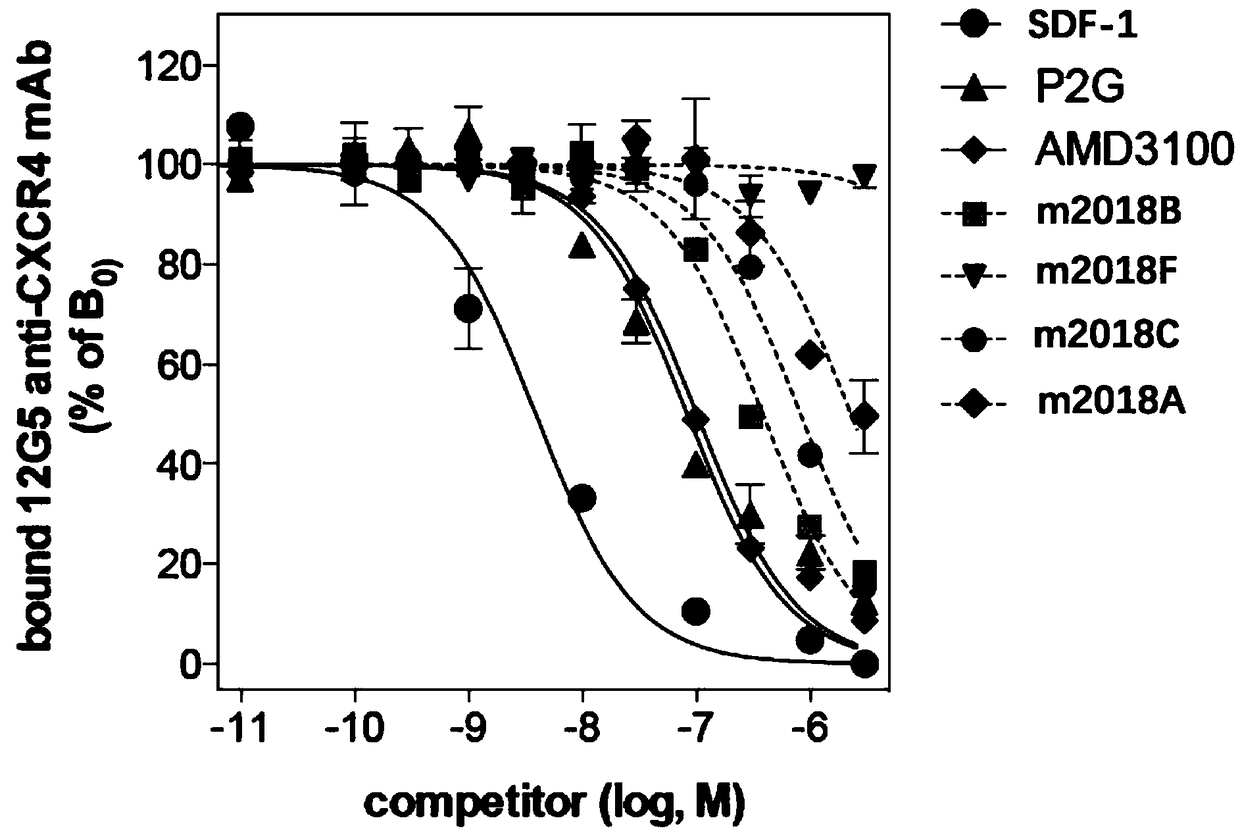
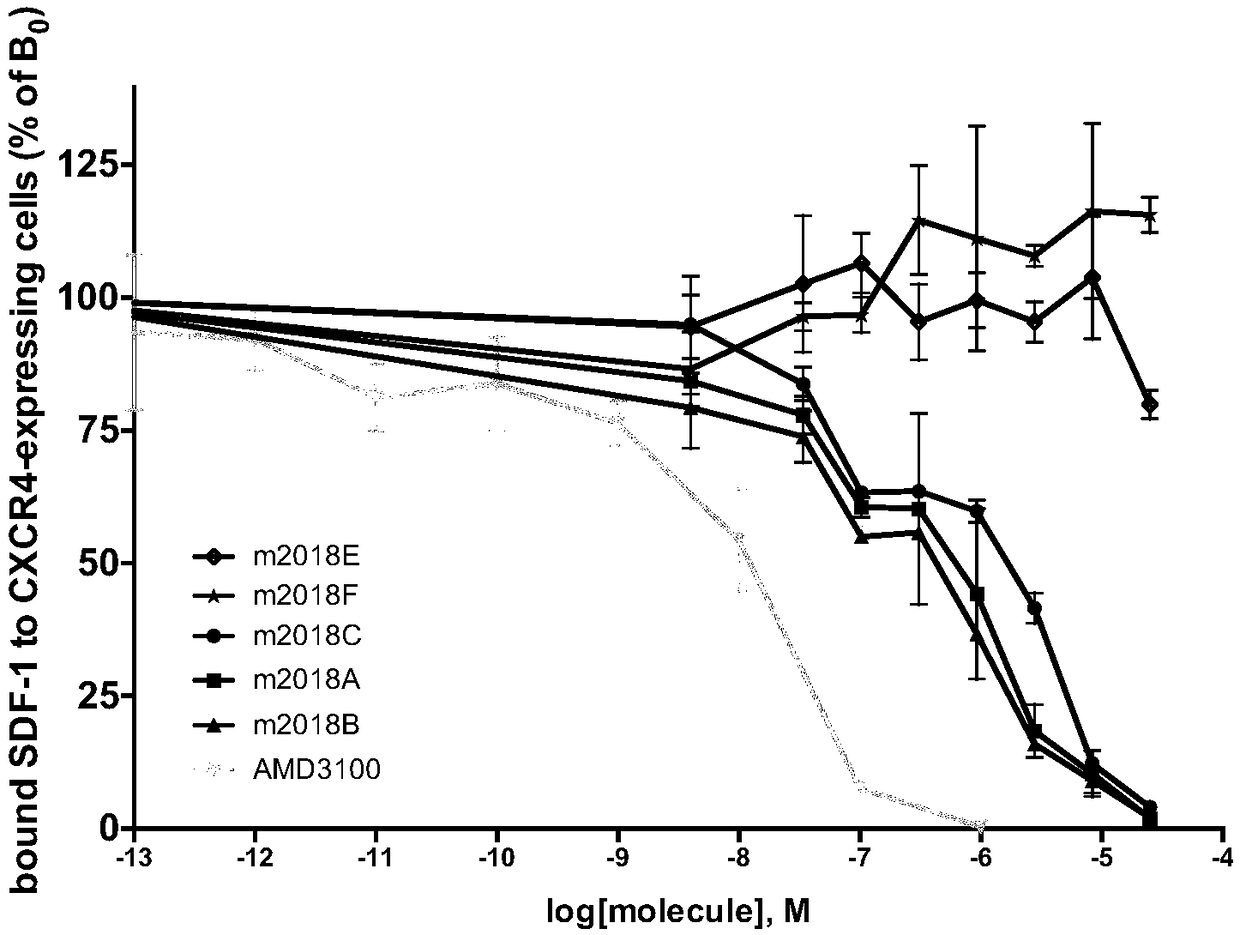
[0054] Experimental Example 1 Inhibition of Test Compounds Binding to 12G5 Antibody CXCR4 Receptor Experiment
[0055] CXCR4 is a cell membrane biomarker of cancer cell proliferation and malignant division, and SDF-1 is a natural chemokine and agonist of CXCR4. Therefore, by effectively inhibiting the binding of CXCR4 to SDF-1, the signal of CXCR4 can be inhibited, normal cell physiological functions can be maintained, and hematopoietic differentiated cells (immune cells and leukocytes) can be prevented from entering the malignant proliferation program. When CXCR4 is stimulated by a signal that is too strong, whether it is positive regulation or negative regulation will cause CXCR4 intracellular signal regulation disorders, resulting in imbalanced cell growth.
[0056] 12G5 is a general commercial antibody that recognizes CXCR4 as a murine monoclonal antibody. 12G5 antibody has strong binding affinity to CXCR4 and can be used to recognize CXCR4.
[0057] In this experiment, ...
experiment example 2
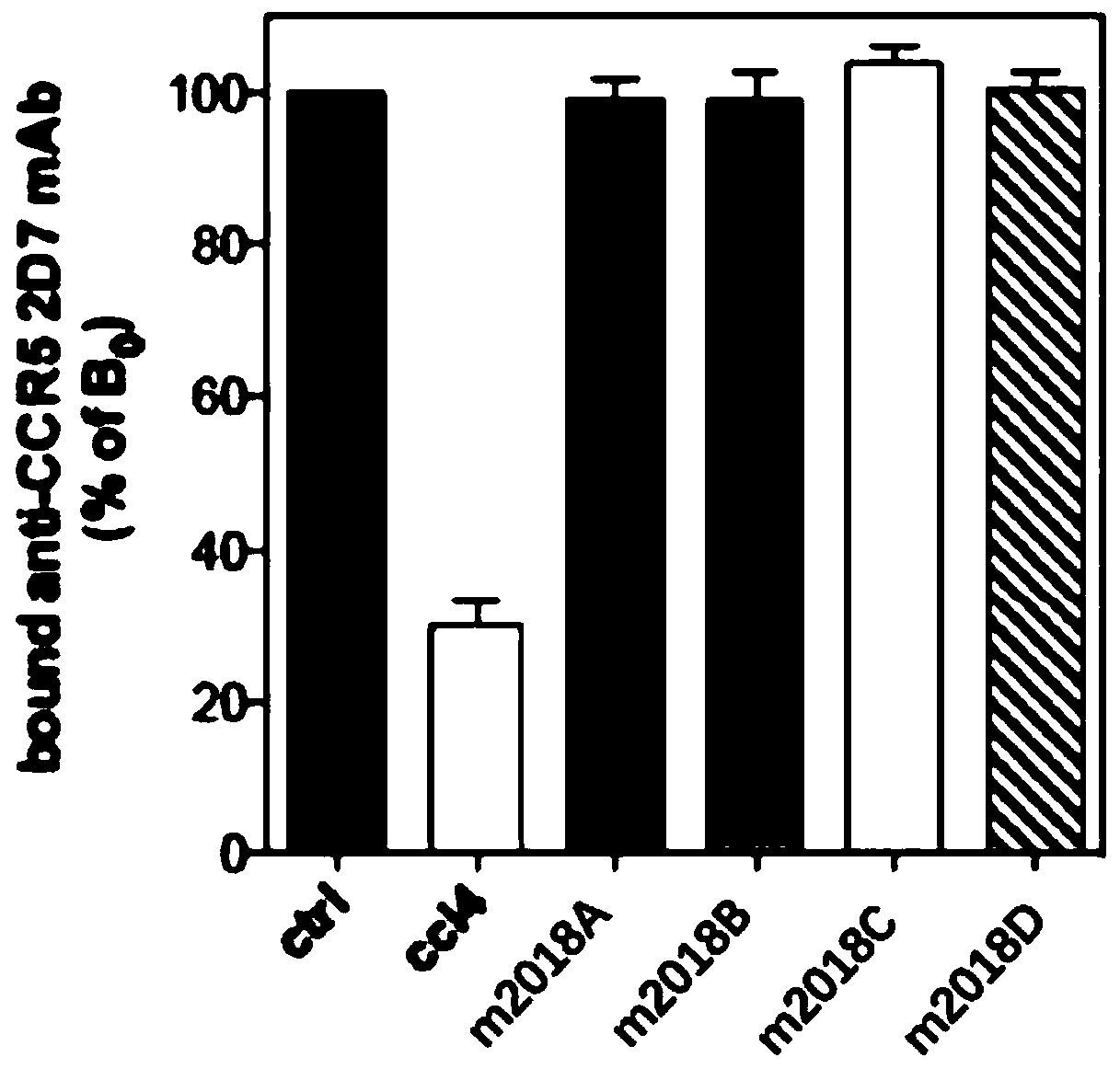
[0066] Experimental example 2 Specific recognition experiment of test compound to CXCR4
[0067] The CCR5 receptor is a specific chemokine receptor expressed by T lymphocytes in the activated state. For T lymphocytes, both CXCR4 and CCR5 are induced to express when they are activated or activated by various signals. CXCR4 and CCR5 sometimes aggregate into heterodimers, and the irregular combination will lead to changes in the signaling of membrane receptors and complete changes in cell physiological functions, which may produce unpredictable side effects, resulting in membrane receptors produced by them. Signaling mutagenic malignancy signaling for carcinogenesis. Therefore, in order to confirm the specific recognition ability of the three lauryl diamine derivatives in the present application to CXCR4, this experiment was carried out.
[0068] In order to confirm the binding specificity of the test compound to CXCR4 but not to CCR5, the inventors chose to use A3.01-R5T lymph...
Embodiment 1
[0071] Example 1), the Kd (dissociation constant) is selected as 2.2nM, that is, the concentration of the CCR5 2D7 antibody is 2.2nM. The reaction system is: mix 50,000 cells with 25 μl buffer + 25 μl, 2.2 nM 2D7 antibody to form a 50 μl volume of antibody receptor cell binding model, and then add from 10 -6 M concentrations of the test compounds (m2018A, m2018B, m2018C, m2018D) and the control drug (CCL4) were incubated at 4° C. for 1 hour.
[0072] b. After incubation for 1 hour, use the eluent (mixed by 0.1 μM EDTA + 1 X PBS buffer + 0.01 μM sodium azide + 1% BSA) to remove the liquid twice, each time at 1200 rpm, Centrifuge for 5 minutes and discard the supernatant. Subsequently, 1 / 1000 dilution (0.1ug / 1ml) of F(ab')goat-anti-mouse IgG (FITC-coupled) provided by Jackson ImmunoResearch was added to the above buffer, mixed evenly, light-shielded, and incubated for 30 minutes. Washing with this eluent / buffer was then repeated twice, and the cells were mixed conventionally t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com