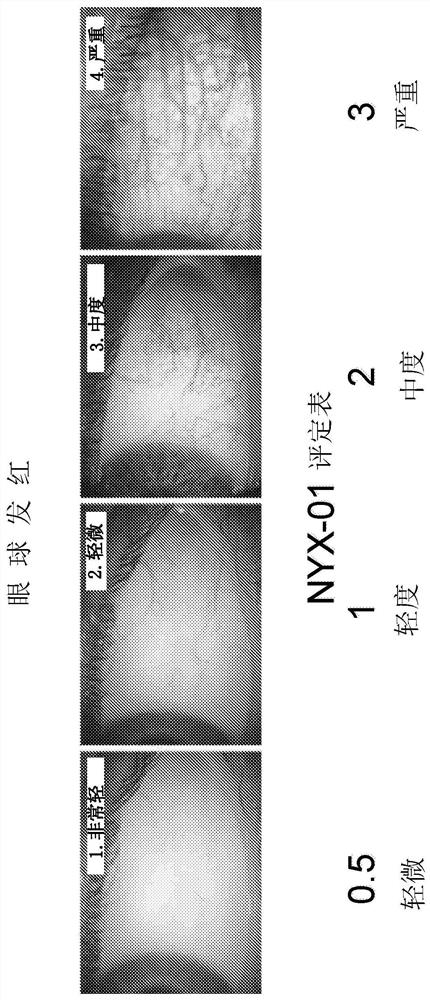
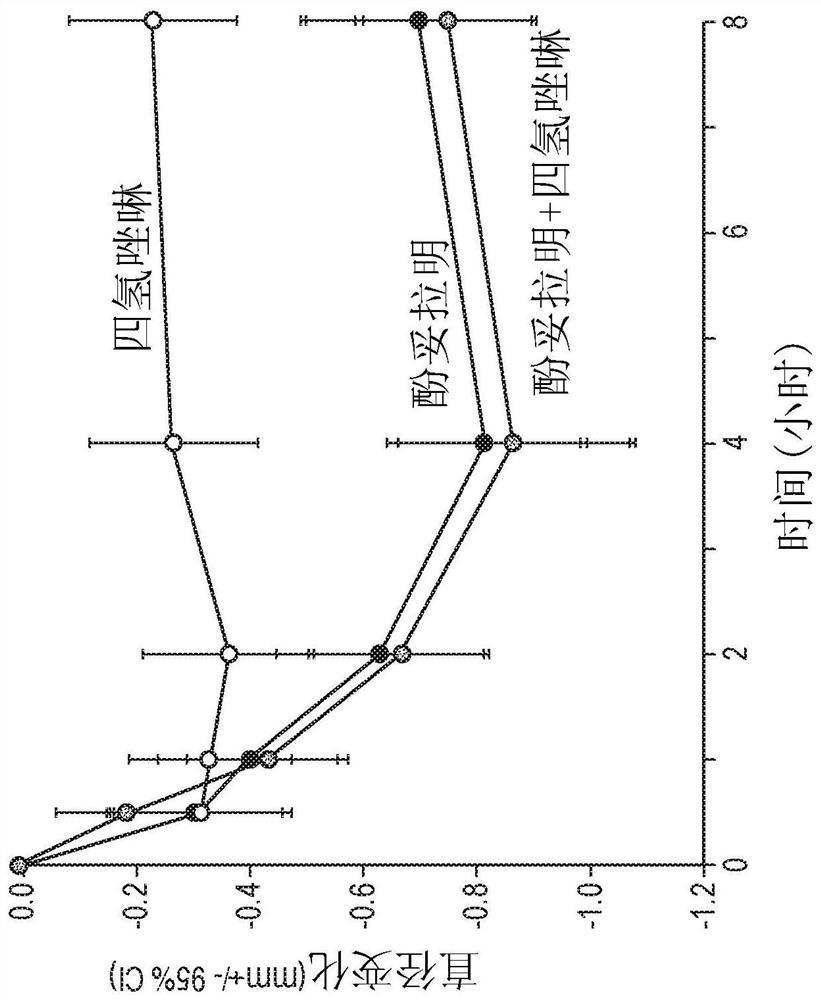
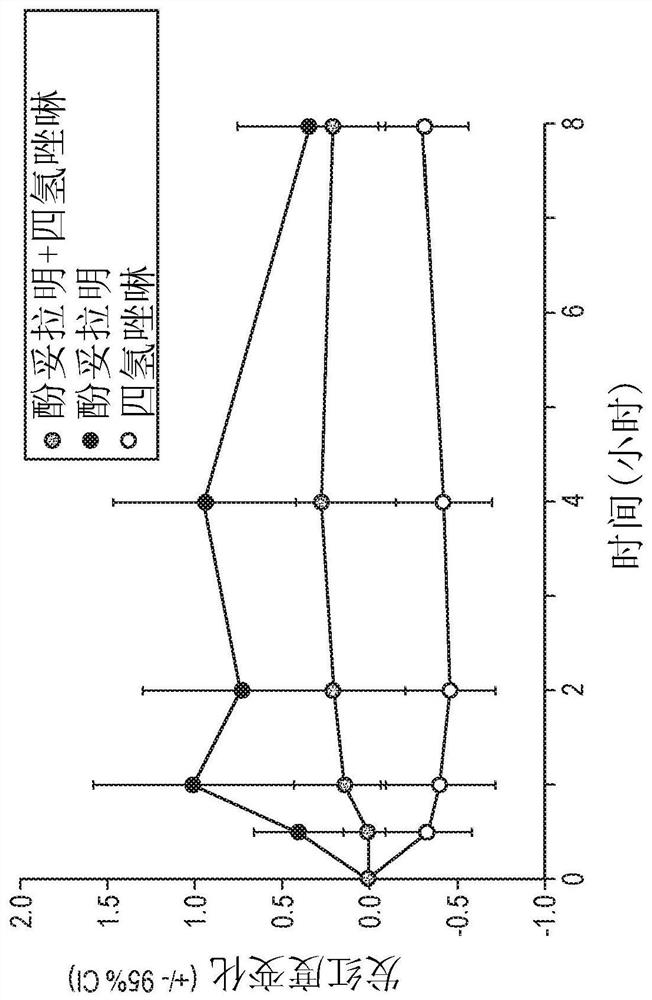
Methods and compositions for treatment of presbyopia, mydriasis, and other ocular disorders
A technology for presbyopia and eyes, which is applied in drug combinations, sensory diseases, muscular system diseases, etc. It can solve patients' discomfort, burning sensation, red eyes and other problems, and achieve the effect of improving visual performance and reducing pupil size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0413] Example 1 - Phentolamine mesylate in the treatment of presbyopia in human subjects
[0414] The therapeutic ability of phentolamine mesylate on human subjects with presbyopia can be evaluated based on clinical studies in which ophthalmic solutions containing phentolamine mesylate are administered to the eyes of patients, The patient is then assessed for improvement in visual performance, including near vision. The experimental procedure and results are described as follows
[0415] Part I - Experimental Procedures
[0416] Human subjects were screened for potential subjects and included in the study if eligible. Exemplary inclusion and exclusion criteria for this study are listed below. If a subject does not meet the inclusion / exclusion criteria, but the investigator believes that the subject should be included in the study, deviations can be tracked after discussions between the Principal Investigator and the study sponsor.
[0417] Inclusion criteria
[0418]...
Embodiment 2
[0436] Example 2 - Phentolamine mesylate in the treatment of presbyopia in human subjects
[0437] The therapeutic ability of phentolamine mesylate on human subjects with presbyopia can be evaluated based on clinical studies in which ophthalmic solutions containing phentolamine mesylate are administered to the eyes of patients, The patient is then assessed for improvement in visual performance, including near vision. The experimental procedure and results are described below.
[0438] Part I - Experimental Procedures
[0439] Human subjects were screened for potential subjects and included in the study if eligible. Exemplary inclusion and exclusion criteria for this study are listed below. If a subject does not meet the inclusion / exclusion criteria, but the investigator believes that the subject should be included in the study, deviations can be tracked after discussions between the Principal Investigator and the study sponsor.
[0440] Inclusion criteria
[0441] · A...
Embodiment 3
[0473] Example 3 - Reversal of mydriasis in human subjects with phentolamine mesylate
[0474] The ability of phentolamine mesylate to reverse pharmacologically induced mydriasis in the eyes of normal healthy human subjects was evaluated in a randomized, crossover, double-blind, placebo-controlled clinical study. Approximately 32 subjects were recruited and randomized 1:1 to one of two treatment sequences. All subjects were first administered a mydriatic (phenylephrine (2.5% w / w) or tropicamide (1% w / w) )). Then, approximately one hour after receiving the mydriatic, subjects were administered study drug according to Treatment Plan 1 or Treatment Plan 2.
[0475] In Treatment 1, subjects received placebo on the first treatment day (Visit 1 / Day 1) and 1 on the second treatment day (Visit 2 / Day 8+2). % w / w Phentolamine Mesylate Ophthalmic Solution. In treatment regimen 2, subjects received 1% w / w phentolamine mesylate ophthalmic solution on the first treatment day (Visit 1 / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com