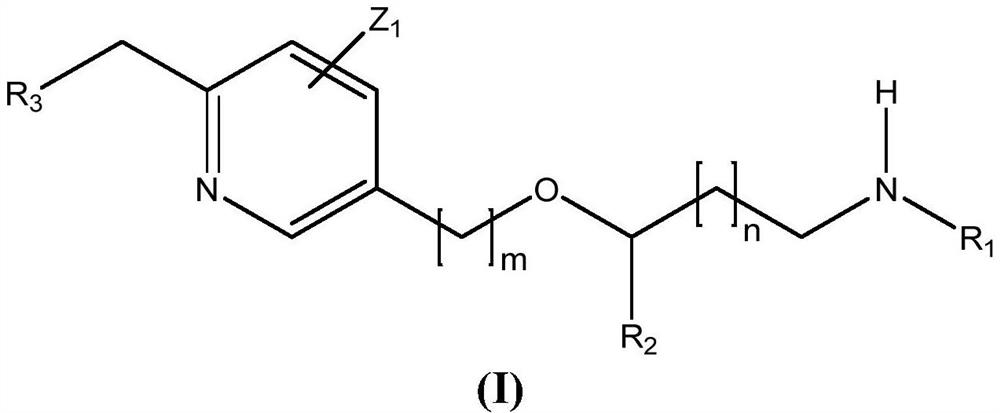
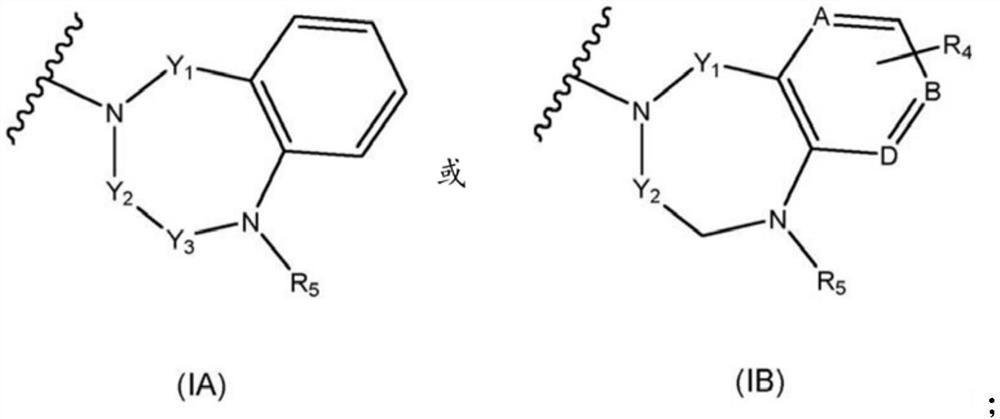
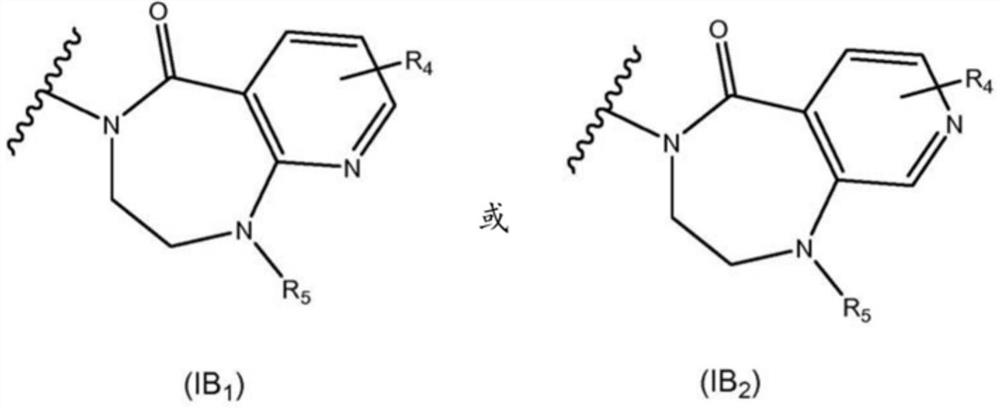
New alcoxyaminopyridine derivatives for treating pain and pain related conditions
A technology of alkoxy group and halogenated alkoxy group, applied in the field of preparing the compound, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0237] In the preparation examples that follow, the preparation of two intermediate compounds as well as compounds according to the invention is disclosed.
[0238] Use the following abbreviations:
[0239] example
[0240] The preparation of examples according to the invention is disclosed.
[0241] Use the following abbreviations:
[0242] Acetonitrile: ACN
[0243] Aq: water-based
[0244] CH: Cyclohexane
[0245] DCM: dichloromethane
[0246] DIPEA: N,N-Diisopropylethylamine
[0247] DMA: N,N-Dimethylacetamide
[0248] EtOAc: ethyl acetate
[0249] h: hour / s
[0250] HPLC: High Performance Liquid Chromatography
[0251] MS: mass spectrometry
[0252] Min: minutes
[0253] Ret: retention time
[0254] rt: room temperature
[0255] Sat: saturated
[0256] TBAI: Tetrabutylammonium iodide
[0257] TFA: Trifluoroacetic acid
[0258] THF: Tetrahydrofuran
[0259] The following methods were used to generate HPLC-MS data:
[0260] Method A: column Eclipse XDB-C1...
example 1
[0262] Example 1: (S)-4-((3-fluoro-5-(3-(methylamino)-1-phenylpropoxy)pyridin-2-yl)methyl)-1-methyl-1, 2,3,4-Tetrahydro-5H-pyrido[2,3-e][1,4]diazepine-5-one.
[0263]
[0264] a) Methyl (S)-5-(3-((tert-butoxycarbonyl)(methyl)amino)-1-phenylpropoxy)-3-fluoropicolinate. To tert-butyl(S)-(3-hydroxy-3-phenylpropyl)(methyl)carbamate (650mg, 2.45mmol) and methyl 3,5-difluoropicolinate (848mg, 4.90 mmol) in DMA (13.6 mL) was added NaH (60% suspension in mineral oil, 147 mg, 3.67 mmol) and the mixture was stirred at rt for 2.5 h. Water was added, extracted with EtOAc, with Na 2 SO 4 Dry, filter and concentrate under vacuum. Purification by flash silica gel chromatography (Gradient: CH to 100% EtOAc) gave the title product (509 mg, 50% yield) as a mixture of two positional isomers (7:3).
[0265] HPLC (Method B): Ret, 5.3 min; ESI + - MS m / z, 419.2 (M+H).
[0266] b) tert-butyl (S)-(3-((5-fluoro-6-(hydroxymethyl)pyridin-3-yl)oxy)-3-phenylpropyl)(methyl)carbamate . To a solu...
example 8
[0276] Example 8: (S)-4-((3-fluoro-5-(3-(methylamino)-1-(thiophen-2-yl)propoxy)pyridin-2-yl)methyl)-1- Methyl-1,2,3,4-tetrahydro-5H-pyrido[2,3-e][1,4]diazepan-5-one.
[0277]
[0278]a) 4-((3,5-difluoropyridin-2-yl)methyl)-1-methyl-1,2,3,4-tetrahydro-5H-pyrido[2,3-e][ 1,4] Diazepan-5-one. To 1-methyl-1,2,3,4-tetrahydro-5H-pyrido[2,3-e][1,4]diazepine-5-one (260mg, 1.46mmol) To a solution in DMF (6 mL) at 0 °C was added NaH (60% suspension in mineral oil, 88 mg, 2.20 mmol), and the mixture was stirred at rt for 30 min. The reaction mixture was cooled at 0 °C, a solution of 2-(chloromethyl)-3,5-difluoropyridine (288 mg, 1.76 mmol) in DMF (5.5 mL) and TBAI (54 mg, 0.147 mmol) were added, and The mixture was warmed to rt and stirred for 20 h. Water was added and extracted with EtOAc. The organic layer was washed with Na 2 SO 4 Dry, filter and concentrate. Purification by flash chromatography (Gradient: CH to 100% EtOAc) gave the title product (395 mg, 88% yield).
[027...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com