Method for prognosis evaluation of infected patients and multi-cytokine detection kit
A detection kit and prognosis evaluation technology, applied in the field of medical testing, can solve the problems of different patient standards, different results, and no unified conclusion, etc., achieve high diagnostic efficiency, simple calculation method, and improve prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
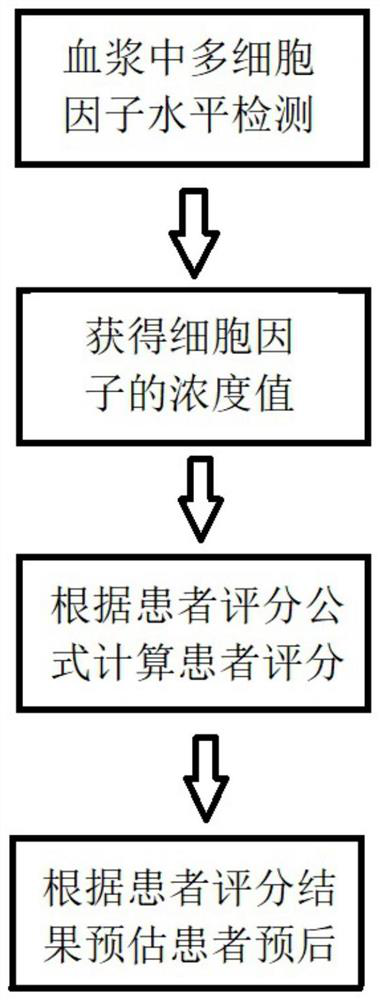
[0042] A patient who was clinically diagnosed as infection was selected, and the patient was divided into 30 days of living group and 30 days of death group according to 30 days, and plasma multi-cytokine level detection was used to detect the cytokine specimens of clinical residual plasma. : IL-1Rα, IL-2Rα, IL-1β, IL-6, IL-16, IL-12, IL-9, MIG, MIP-1α, MIP-1β, IFN-α2, SDF-1α, LIF, TNF -α, TNF-β, MIF, RANTES, CTACK, M-CSF, HGF, SCF, TRAIL, EOTAXIN, PDGF-BB, IP-10, SCGF-β. The above plasma cytokine level is incorporated into single factor Logistic regression. The results showed that IL-1Rα, IL-16, LIF, HGF, SCF, M-CSF and CTACK were associated with 30 days prognosis (P <0.05); P <0.05 was analyzed by a subject working characteristic curve (ROC) curve. The plasma cytokine level is converted to a two-class variable and incorporates multiple factors Logistic regression, resulting in a variable associated with the patient's 30 days prognosis. The proposed independently related variable...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com