Clostridium difficile multi-component vaccine
A Clostridium difficile and Clostridium difficile toxin technology, applied in the field of vaccine administration and Clostridium difficile vaccine, can solve the problems of reduced recurrence rate of CDI and low effectiveness of anti-hypertoxic Clostridium difficile strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0114] Preparation of immunogenic compositions
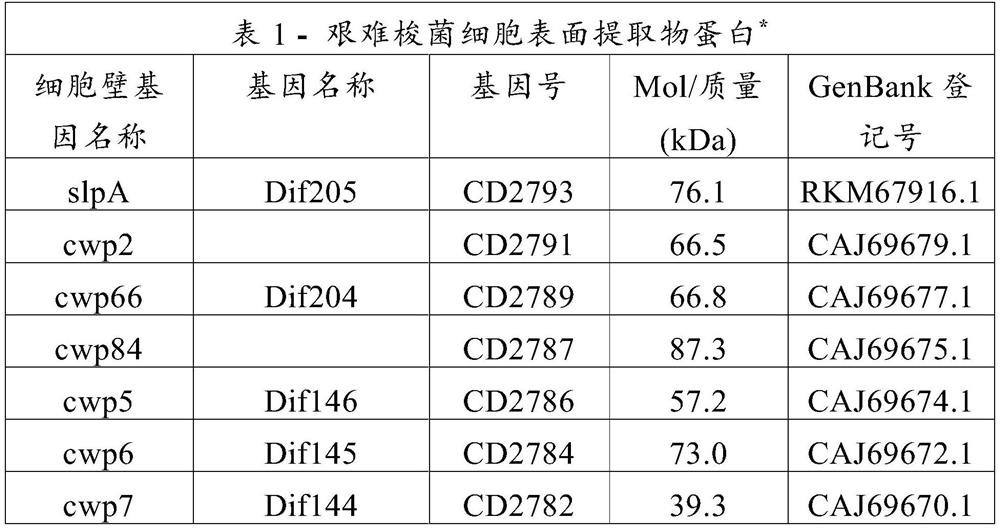
[0115] The immunogenic composition according to the invention can be prepared by simply admixing component (a) which is, for example, a suspension of inactivated whole cells of Clostridium difficile or a CSP preparation with component (b) (eg resuspended particles), the component (b) is eg a solution of at least one toxin A or B toxoid and / or at least one non-toxic C. difficile toxin A or toxin B polypeptide. Appropriate ratios of components (a) and (b) can be determined by a person skilled in the art. In certain embodiments, sufficient inactivated whole cell (WC) or cell surface extract (CSE) component (a) should be used to provide 10 7 -10 11 cells or 10-100 μg of CSE; and enough toxoid / toxin polypeptide fragment component (b) should be used to provide 4-500 μg of toxoid or non-toxic polypeptide fragment. Other amounts may be used if multiple immunizations are envisaged. The amounts of the components can be adjusted inde...
Embodiment 1
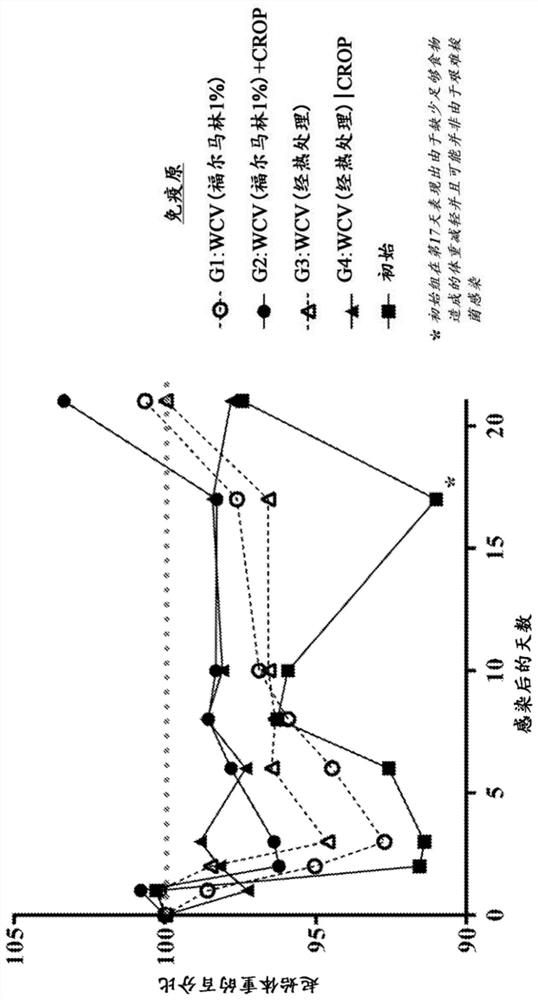
[0170] A sample of Clostridium difficile BI / NAP1 / 027 strain was obtained from the American Type Culture Collection, Manassas, VA ( BAA-1870) obtained. The glycerol stock was used to inoculate a 50 mL brain heart infusion-L-cysteine (BHI-Cys) growth medium starter culture (BHI-BD Bacto #237200) grown overnight at 37 °C for 17 hours under anaerobic conditions. ; L-cysteine-Sigma #168149). Optical density (OD 600 ) read and contamination test. Inoculate 1 L of freshly reduced BHI-Cys medium with starter culture to an OD of 0.1 600 and incubated anaerobically at 37°C until an OD of about 1.0 was reached 600 (5-6 hours of growth). Cells were harvested by centrifugation (6000 rpm, 20 min, 4°C) and washed three times with IX PBS. After the last wash, the pellet was resuspended in 1X PBS and serial dilutions were prepared to determine the number of colony forming units (CFU) by plating; 600 Readout for cell counts. The pellet suspension was divided into two equal volumes fo...
Embodiment 2
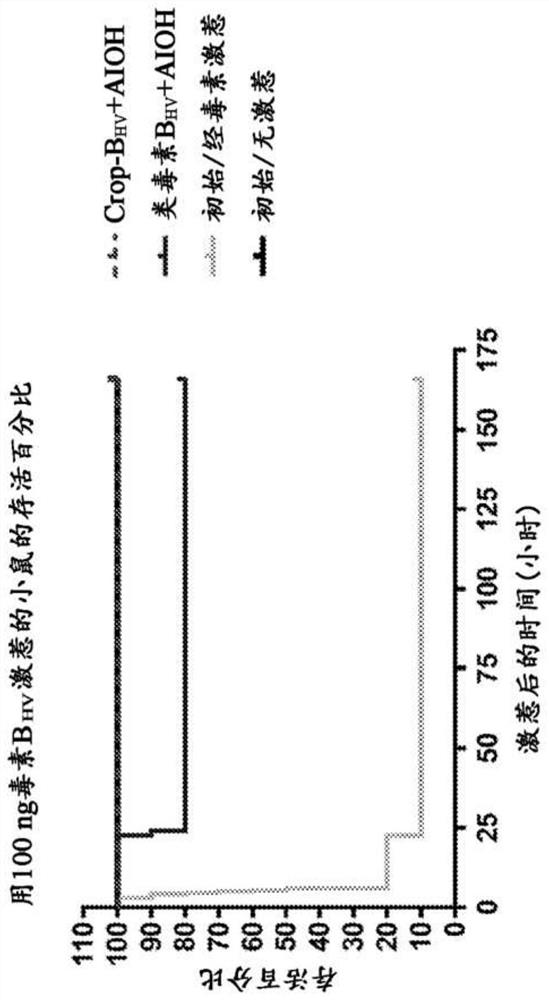
[0179] In experiments evaluating protection against toxin BHV challenge, CROPB HV The immunized mice were completely protected from toxin challenge (10 out of 10 mice), whereas toxoid B HV Eight out of 10 mice immunized were protected from toxin challenge (Table 3; figure 2 ). These data suggest that CROPB HV is the preferred antigen, equivalent to or superior to full-length inactivated toxin B HV .
[0180]
[0181]
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com