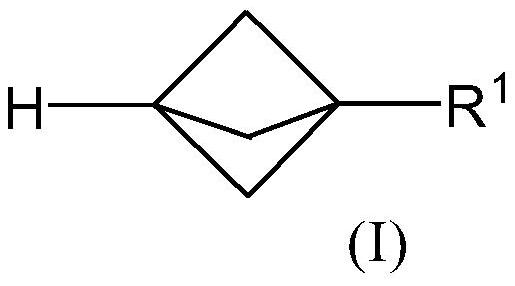
Propellane derivates and synthesis
A technology of alkyl compounds, applied in the field of synthetic organic chemistry, which can solve the problems of reagent availability and cost constraints for commercial use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1: General process
[0140] Preparation of anhydrous EtOH, anhydrous MeOH or anhydrous MeOH and anhydrous ET 2 O ~ 2: 1 mixture of Fe (ACAC) 3 Solution (30 mol% or 50 mol%) and in N 2 The mixture was stirred for 2 min. Add [1.1.1] rampane (1 equivalent, for dissolving in etha) 2 O solution) and suitable reagents (1.2-3 equivalents) capable of providing all or part of the substituent, then add phsih 3 (1.0-1.5 equivalents). After stirring at room temperature (RT) overnight, a mixture containing the product was concentrated to give the compound, which can be further purified by flash chromatography on silica gel, or non-separated or non-purified product, continued to treat the product to produce a corresponding derivative .
Embodiment 2
[0141] Example 2: Double ring [1.1.1] pentane-1-methitrile:
[0142]
[0143] According to the general process, 4-methylbenzenesulfin cyanocyanide (TSCN) is used as a suitable reagent capable of providing all or some of the substituents (1.1.1] pentane-1-methitrile. 1 H NMR (400MHz, MeOH-D 4 Δ2.40 (S, 1H), 2.31 (S, 6H).
Embodiment 3
[0144] Example 3: Double ring [1.1.1] pentane-1-ylomethyme
[0145]
[0146] By dissolving the double ring [1.1.1] pentane-1-methitrile in anhydrous ET 2 O (0.090 m) was prepared for bicyclic [1.1.1] pentane-1-ylomethynine and cooled to 0 ° C. The LaH solution (5.3 equivalent) dissolved in THF was added dropwise by stirring. After 30 min, the solution was quenched at 0 ° C by adding EtOAc (2 mL). The mixture was concentrated to 30% of its original volume and loaded to Si-p-p-toluenesulfonic acid resin column. Rinse the column with MeOH, then use 1N NH dissolved in MeOH 3 Element elution compound. The solution was concentrated to 10% of its original volume, and then acidified with 4 NHCl dissolved in dioxane and evaporated to give a double ring [1.1.1] pentane-1-ylmethylamine. 1 H NMR (400MHz, MeOH-D 4 Δ2.97 (S, 2H), 2.57 (S, 1H), 1.90 (S, 6H); LC / MS (APCI) M / Z 98.1 [C 6 Hide 11 N + h] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com