African swine fever virus cd2v truncated protein and its application in the preparation of wild virus and natural attenuated virus detection kits
An African swine fever virus and truncated protein technology, applied in the biological field, can solve the problems of unclear specificity, no serum antigen protein, etc., and achieve the effect of strong clinical practicability, high protein purity, and good detection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
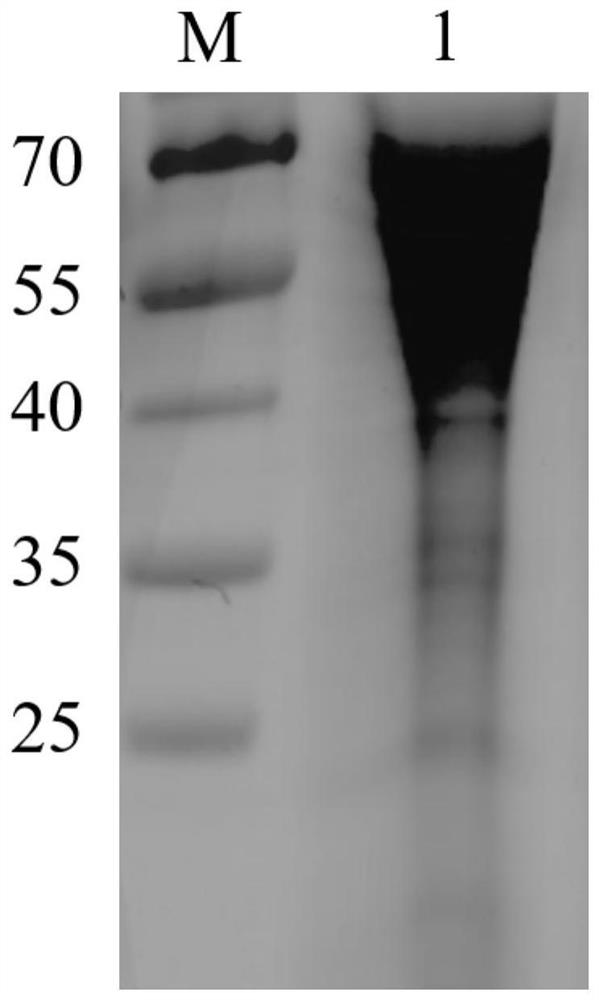
[0028] Obtaining of African swine fever virus truncated protein:
[0029] 1. Obtaining and identification of recombinant plasmids
[0030] The amino acid positions of the N-terminal region and the C-terminal region of the CD2v protein of the African swine fever HLJ strain (GenBank accession number: MK333180.1) were analyzed. Since the expression process of the CD2v N-terminal region requires glycosylation modification, therefore It needs to be expressed in a eukaryotic system, but the CD2vC-terminal region does not require glycosylation modification, so a prokaryotic expression system can be selected for expression. Codon optimization is a key technical means to achieve high-efficiency expression of heterologous proteins, and protein expression is a systematic project, so when performing codon optimization, only replacing codons with the most frequently used codons of a certain species may not be effective. For the best results, it is necessary to consider adjusting GC conten...
Embodiment 2
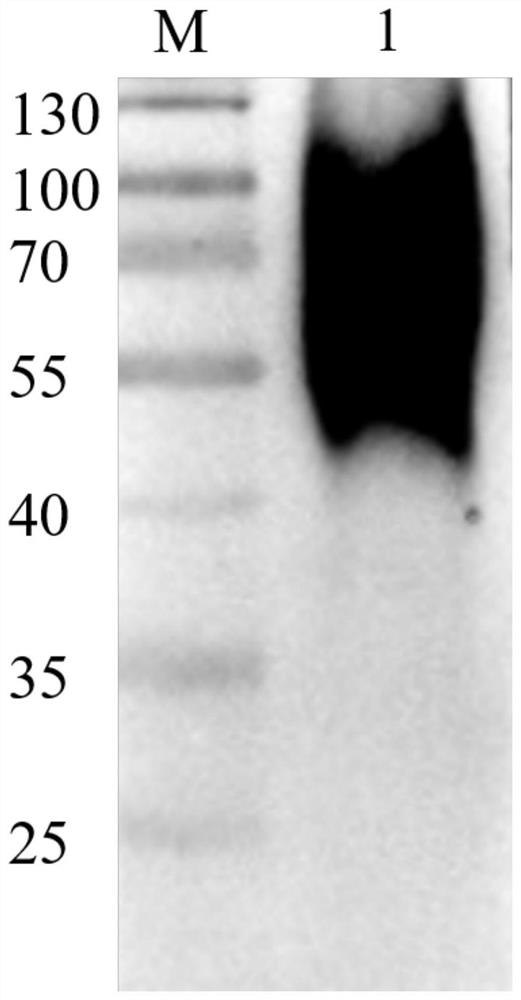
[0041] African swine fever virus CD2v truncated proteins with different expressions are used to distinguish ASFV wild virus and natural attenuated virus:
[0042] Using the method in Example 1, the applicant expressed and purified the eukaryotic expression protein N1 in the N-terminal region of African swine fever virus protein CD2v and the prokaryotic expression protein in the C-terminal region. The prokaryotic-expressed protein and eukaryotic-expressed protein were respectively used as coating antigens to detect clinically known background clinical sera (10 positive sera from wild strain infection, 10 negative sera, and 10 natural attenuated strain infection sera).
[0043] Wherein the P54 antibody positive control and negative control sample preparation methods:
[0044] Referring to the African swine fever virus indirect ELISA antibody detection kit (application number: Example 2 in 202010700335.X), operate according to Example 3 in the patent document.
[0045] CD2v anti...
Embodiment 3
[0062] Construction of African swine fever virus indirect ELISA antibody detection kit (CD2v coating antigen):
[0063] 1. Preparation of positive control:
[0064] For the preparation method of positive control, refer to the preparation method of CD2v antibody positive serum in Example 1.
[0065] 2. Preparation of negative control:
[0066] For the preparation method of negative control, refer to the preparation method of CD2v antibody negative serum in Example 1.
[0067] 3. Preparation of ELISA plate
[0068] The optimal coating concentration of recombinant antigen N1 was determined by square array method to be 50ng / well, and coated at 2-8°C for 14 hours. Discard the coating solution, add 1% bovine serum albumin (BSA) as a blocking solution, and block for 2 hours at 37°C. Discard the blocking solution, air-dry naturally, and then vacuum-pack it into a finished kit enzyme plate.
[0069] 4. Preparation of other components of the kit
[0070] Coating solution: take sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com