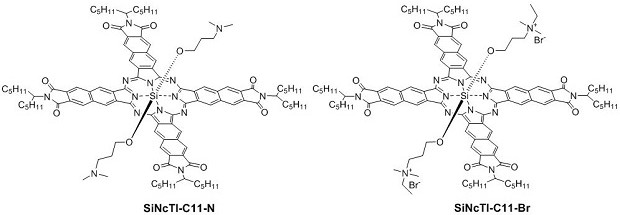
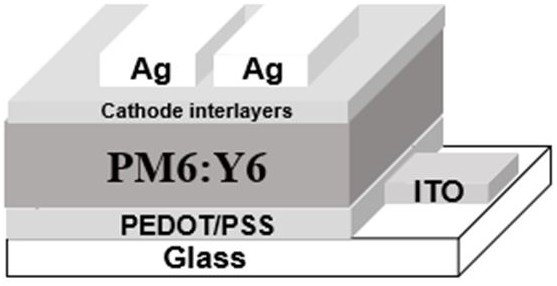
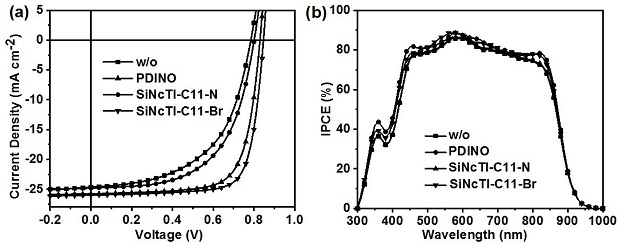
A kind of silicon naphthalocyanine cathode interface material and preparation method and application thereof
A cathode interface and naphthalocyanine technology, which is applied in the field of silicon naphthalocyanine cathode interface materials and its preparation, can solve the problems of limiting the application of naphthalocyanine compounds, poor electron acceptance ability, and high LUMO energy level, and is conducive to large-scale Large production, lower work function, beneficial to the effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The synthesis of compound 2a (amination of 6,7-dicyano-n-butyl-naphthalene monoimide (compound 1a)) is as follows:
[0070]
[0071] Under argon protection, compound 1a (1 g, 3.30 mmol), NaOCH was added to a 100 mL two-necked flask 3 (178 mg, 3.30 mmol), NH 3 (7M in CH 3OH, 33.0 mmol), methanol (30 mL), reacted at 65 °C for 45 min, and cooled to room temperature. The reactant was settled in 45 mL of water and filtered with suction to obtain a white solid. Column separation was carried out with a mixed solvent of dichloromethane and methanol (25 / 1, v / v) as the eluent to obtain a white solid 2a with a yield of 695 mg and a yield of 66.08%.
[0072] 1 H NMR (400MHz, CDCl 3 )δ8.35(d,4H),4.23(m,2H),2.12(m,2H),1.23(m,2H),0.81(m,3H)ppm.
[0073] 13 C NMR (101MHz, d 6 -DMSO)δ167.7,137.66,137.3,134.1,134.2,129.4,129.2,126.4,125.6,124.4,123.4,52.3,26.4,22.1,14.1ppm.
[0074] HRMS(MALDI-TOF):Calcd for C 18 H 16 N 4 O 2 Exact Mass: 320.1273, found: 320.1245 (M + )...
Embodiment 2
[0076] The synthesis of compound 2b (amination of 6,7-dicyano-(6-undecyl)naphthalene monoimide (compound 1b)) is as follows:
[0077]
[0078] Under argon protection, compound 1b (1 g, 2.49 mmol), NaOCH was added to a 100 mL two-necked flask 3 (269 mg, 4.98 mmol), NH 3 (7M in CH 3 OH, 24.9 mmol), methanol (30 mL), reacted at 65 °C for 30 min, and cooled to room temperature. The reactant was settled in 30 mL of water and filtered with suction to obtain a white solid. Column separation was carried out with a mixed solvent of dichloromethane and methanol (50 / 1, v / v) as the eluent to obtain a white solid 2b with a yield of 711 mg and a yield of 68.11%.
[0079] 1 H NMR (400MHz, CDCl 3 )δ8.38(d,J=19.3Hz,4H),4.26(m,1H),2.09(m,2H),1.74(m,2H),1.24(m,12H),0.81(m,6H)ppm .
[0080] 13 C NMR (101MHz, d 6 -DMSO)δ167.8,137.8,137.4,134.2,134.0,129.3,129.2,126.5,125.9,124.8,123.9,52.1,32.1,31.3,26.1,22.4,14.2ppm.
[0081] HRMS(MALDI-TOF):Calcd for C 25 H 30 N 4 O 2 Exact Mas...
Embodiment 3
[0082] Example 3: Synthesis of Compound 2c
[0083]
[0084] Under argon protection, compound 1c (0.81 g, 2.00 mmol), NaOCH was added to a 100 mL two-necked flask 3 (538 mg, 10 mmol), NH 3 (7M in CH 3 OH, 2 mmol), methanol (30 mL), reacted at 50 °C for 30 min, and cooled to room temperature. The reactant was settled in 30 mL of water and filtered with suction to obtain a white solid. Column separation was carried out using a mixed solvent of dichloromethane and methanol (50 / 1, v / v) as eluent to obtain a white solid 2c with a yield of 510 mg and a yield of 60.15%.
[0085] HRMS(MALDI-TOF):Calcd for C 26 H 24 N 4 O 2 Exact Mass: 424.1899, found: 424.2860 (M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com