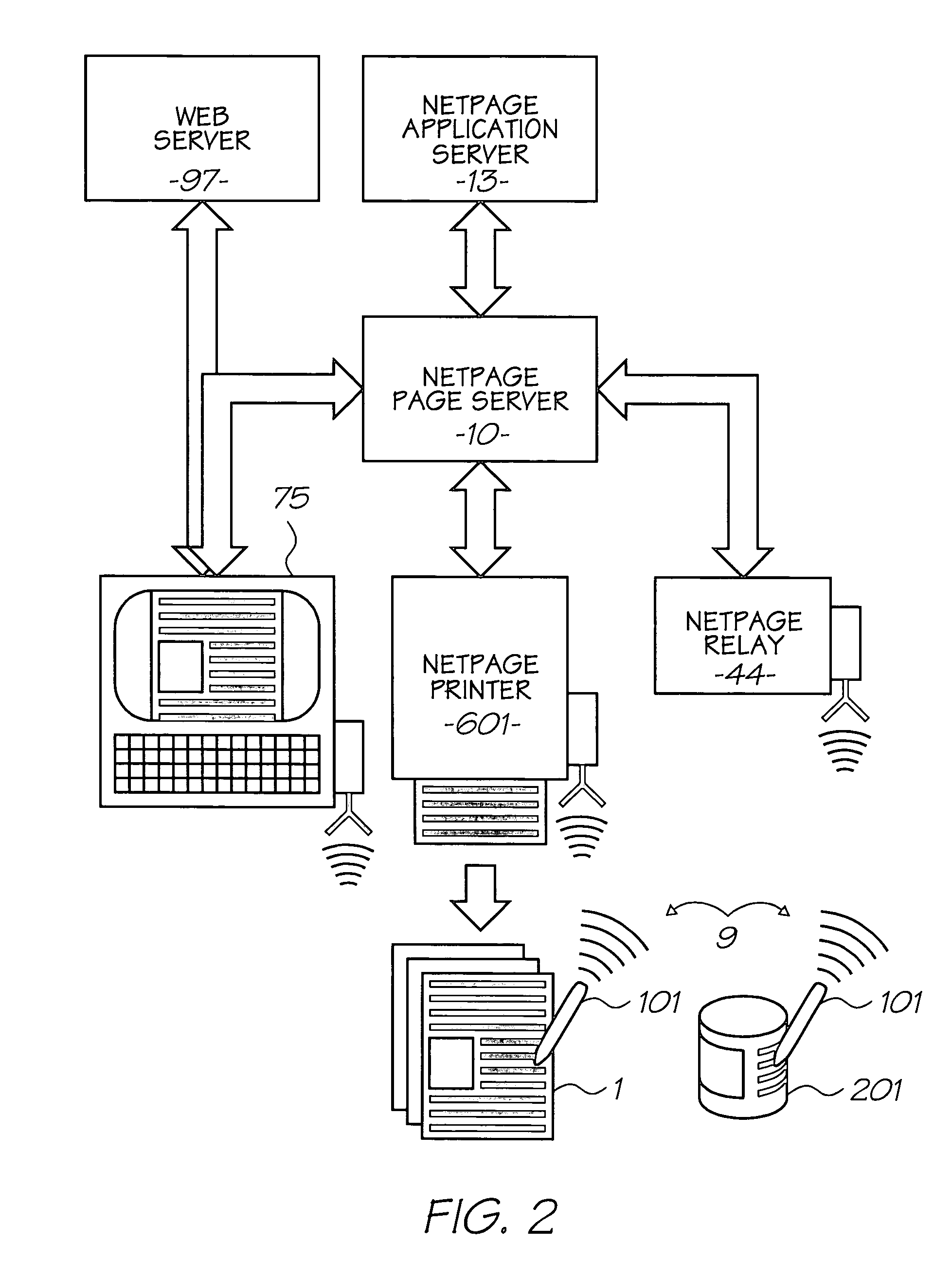
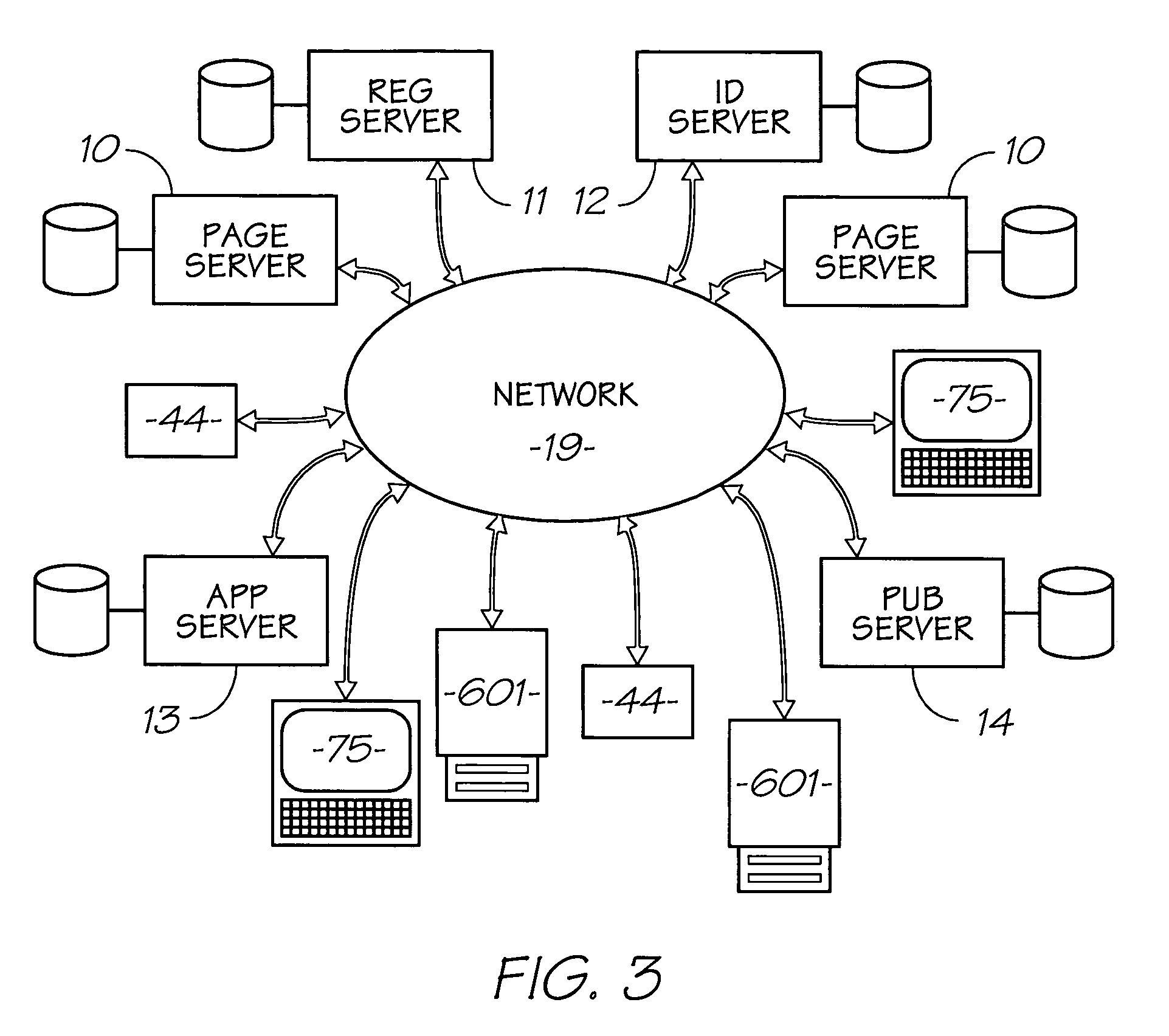
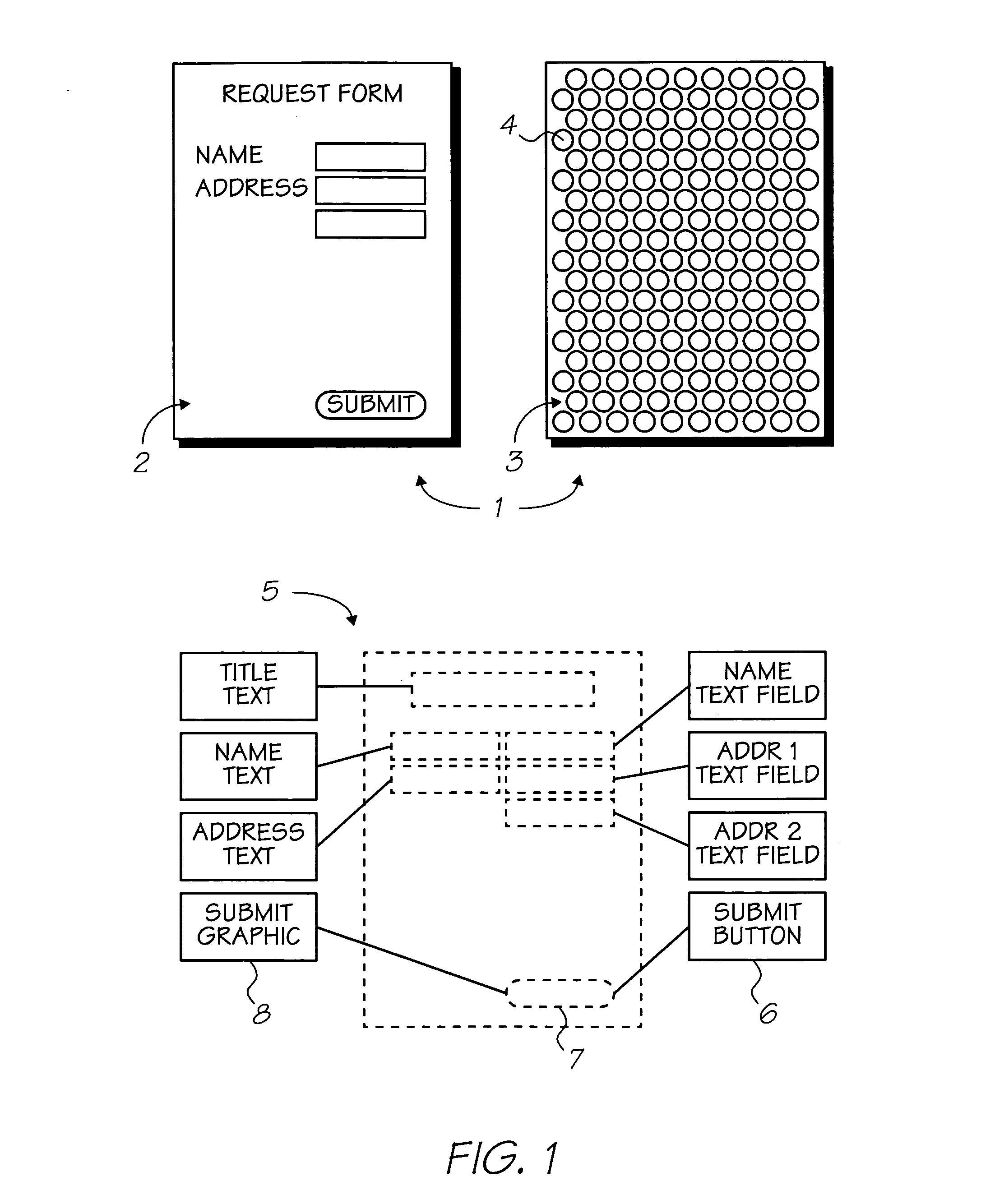
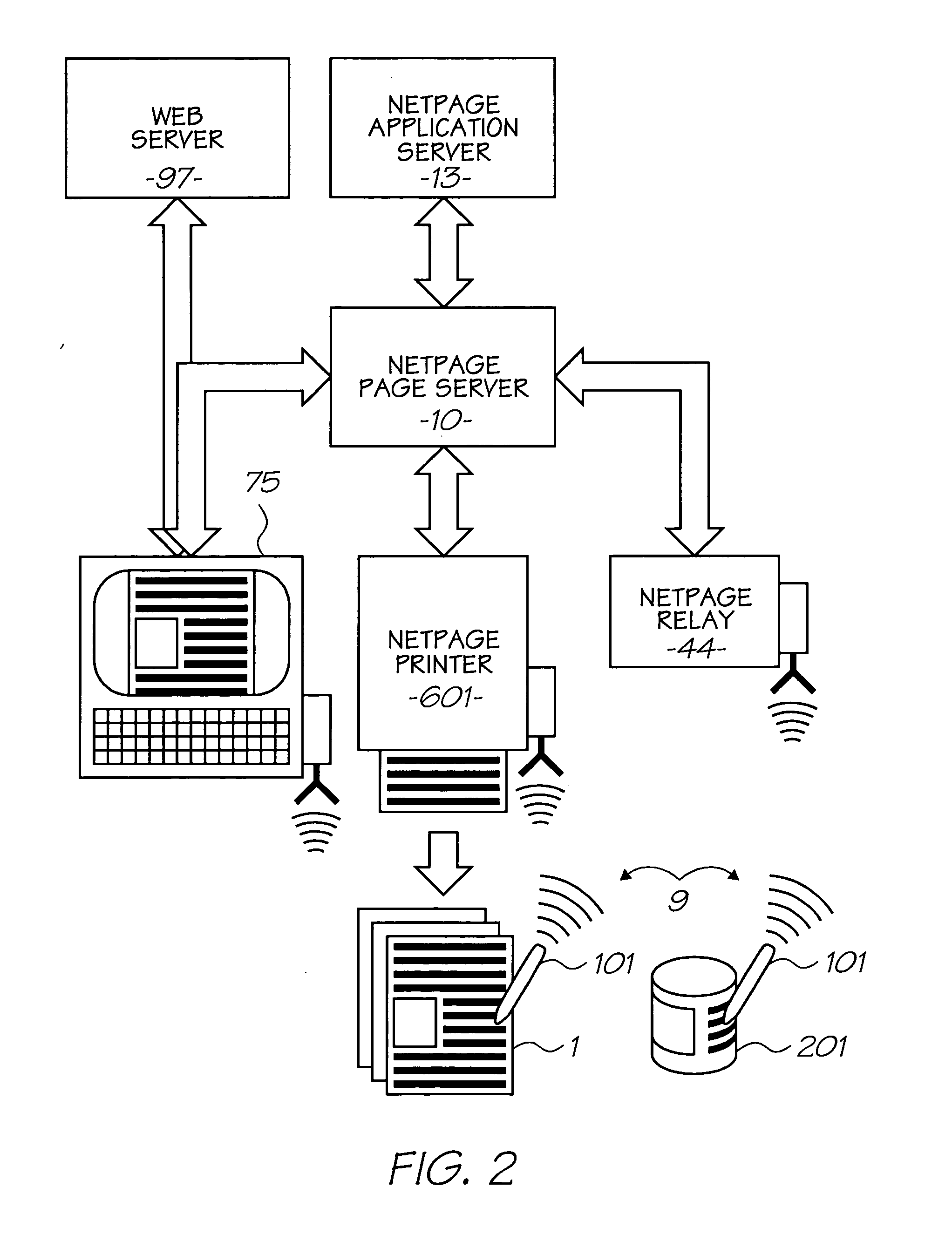
Patents
Literature
72 results about "Naphthalocyanine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
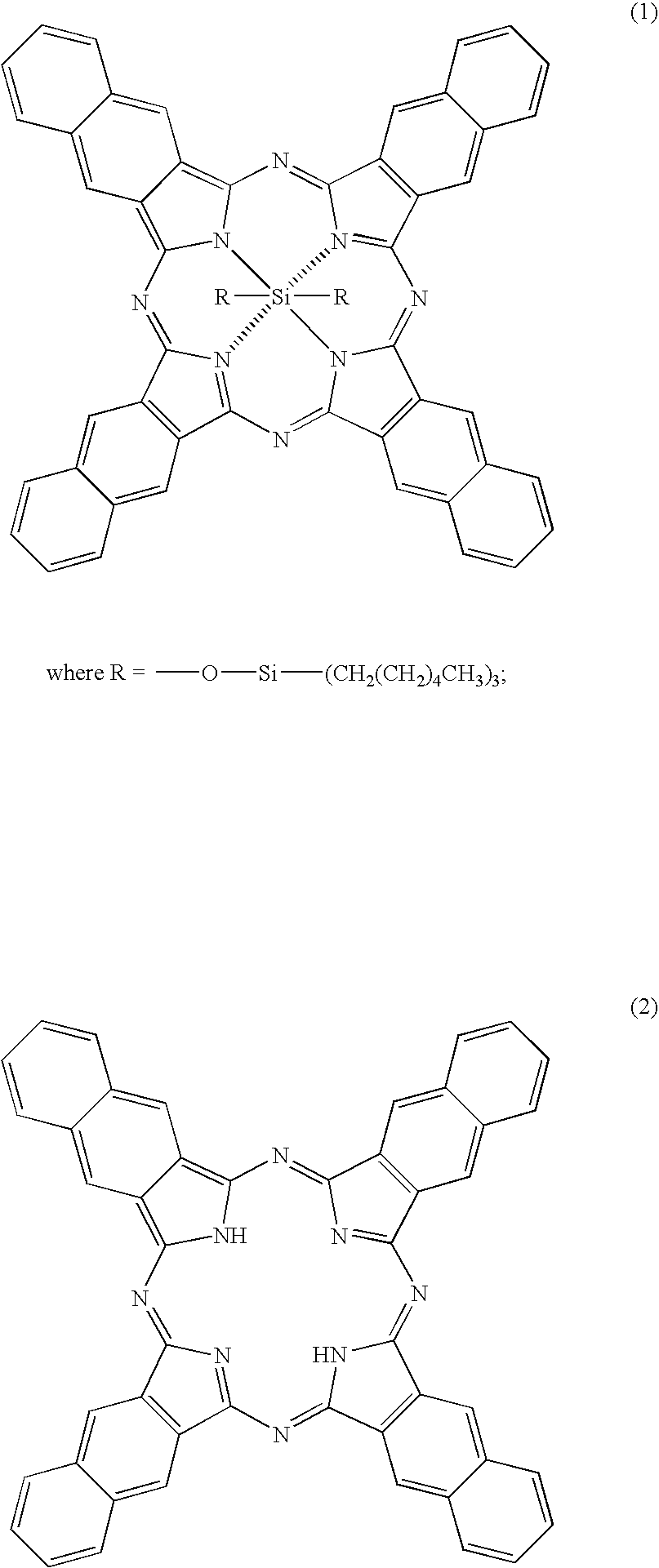
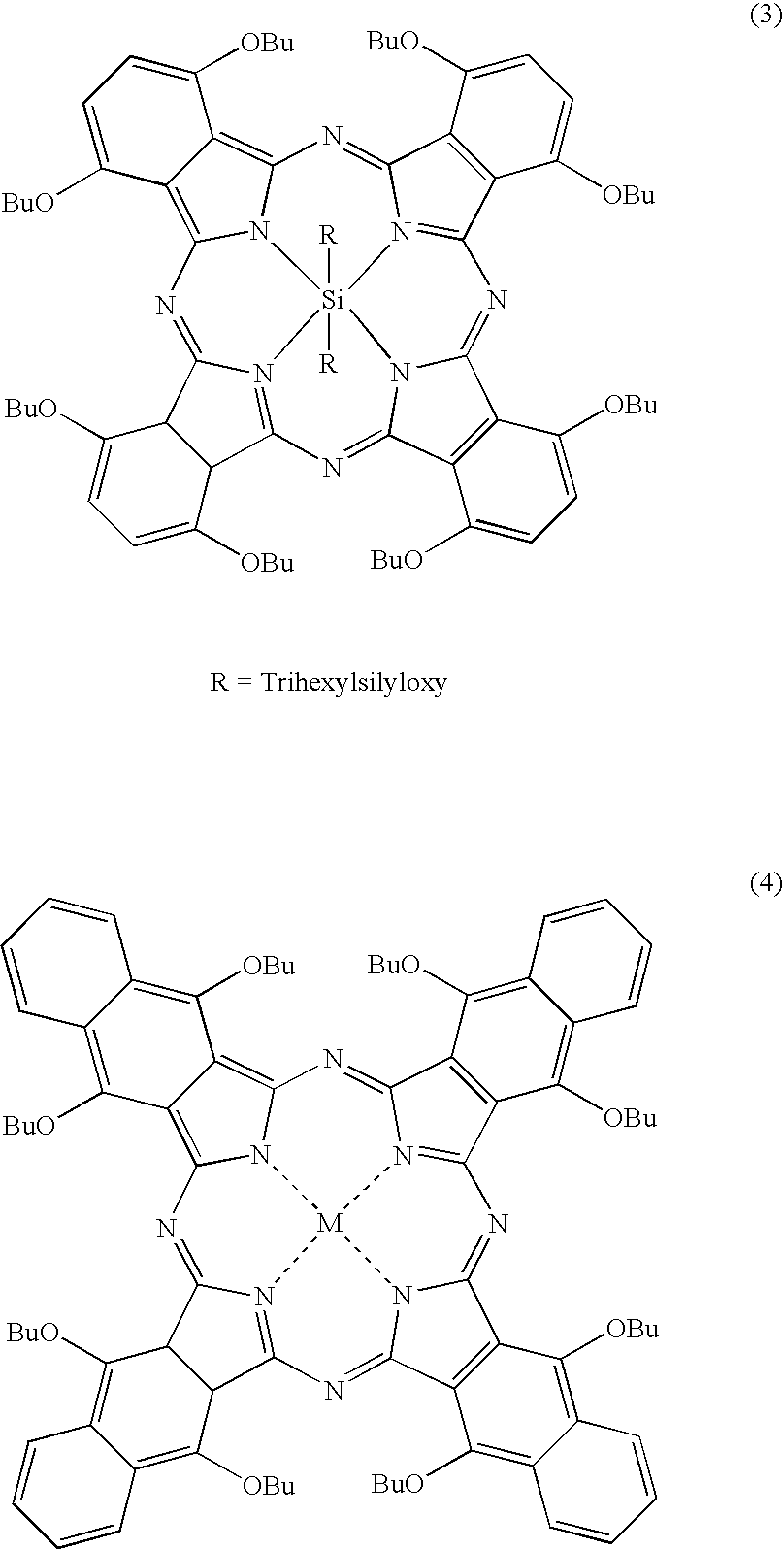
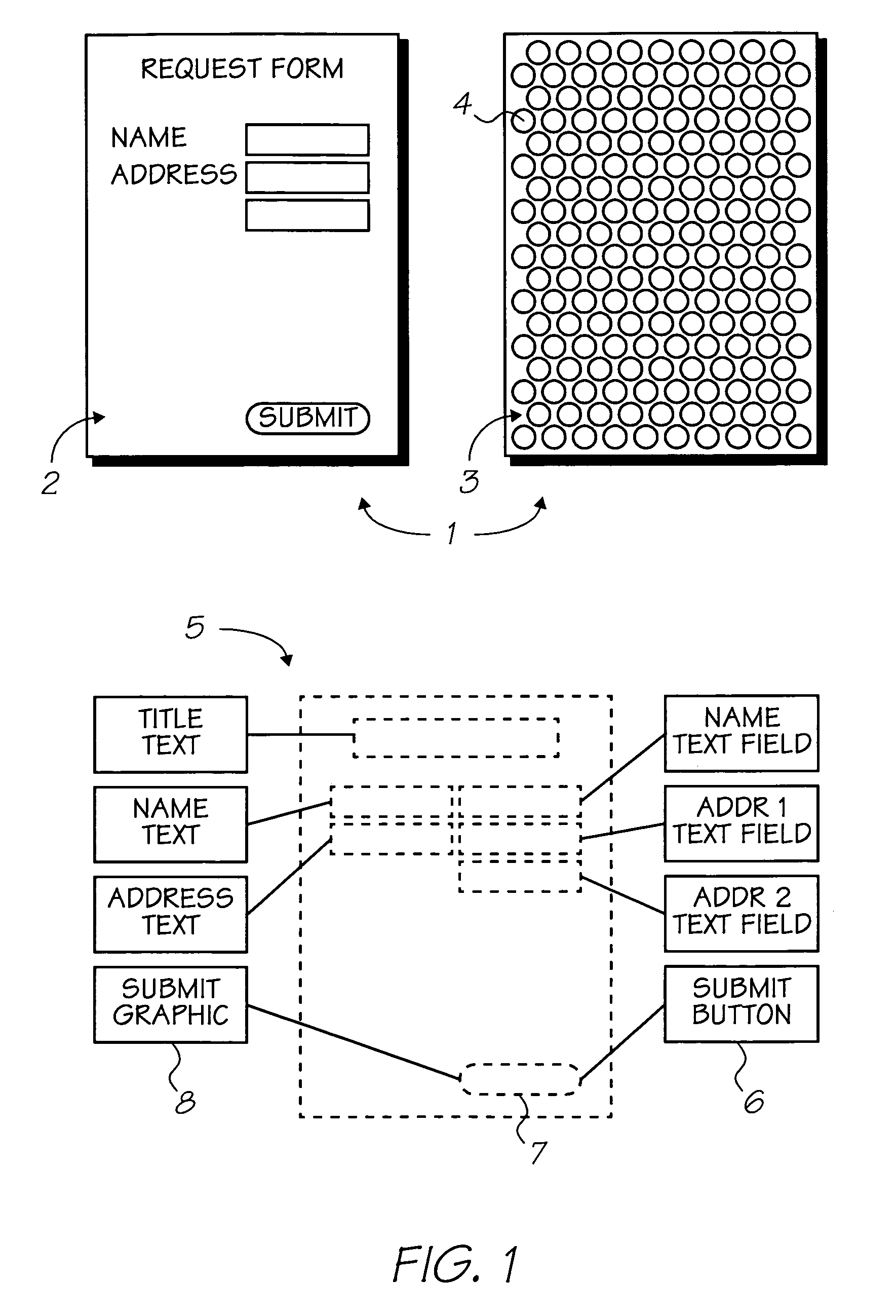
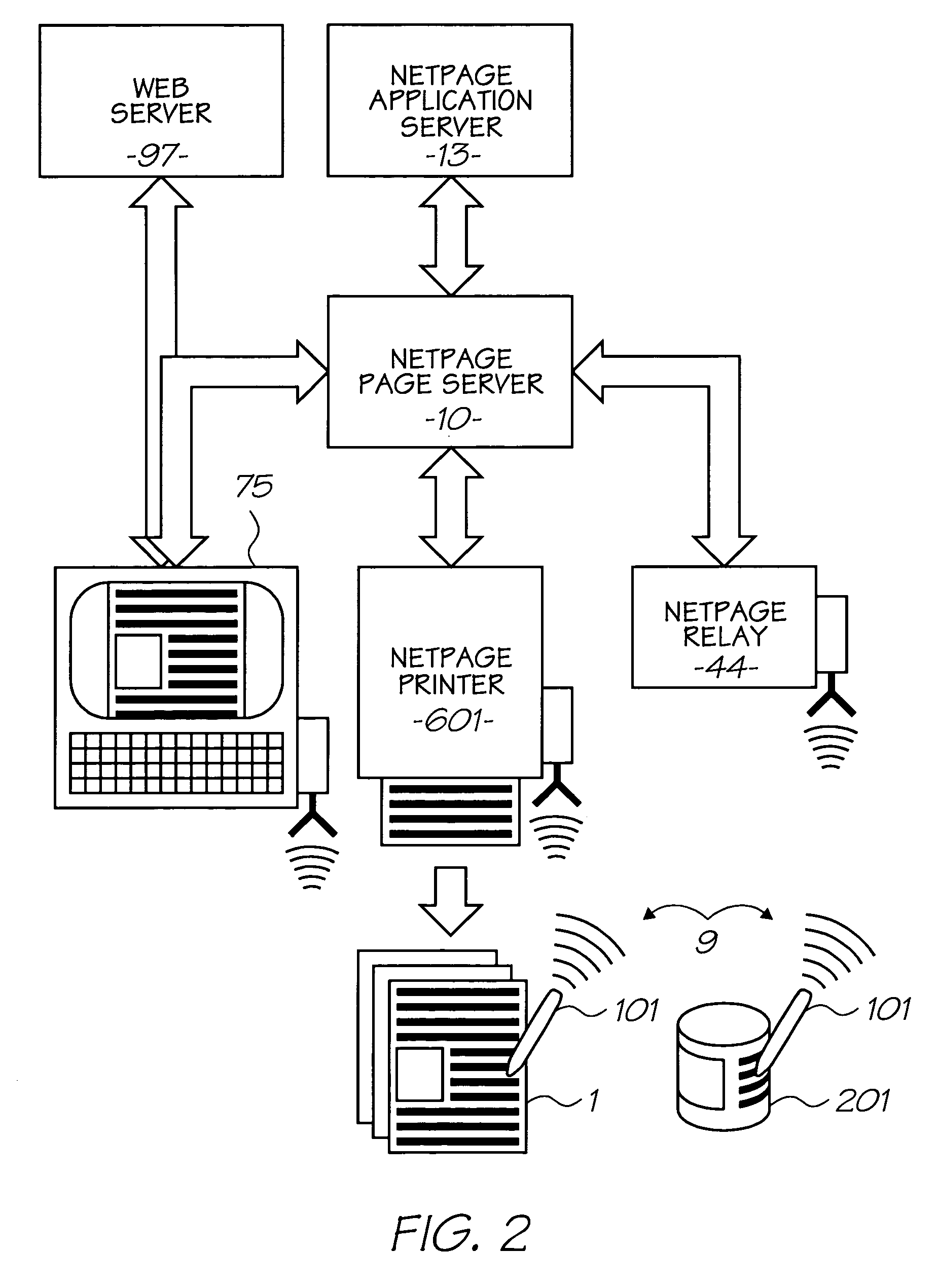
Naphthalocyanine is a cross-shaped organic molecule consisting of 48 carbon, 8 nitrogen and 26 hydrogen atoms, it is a derivative of phthalocyanine. IBM Research labs used it for developing single-molecule logic switches and visualizing charge distribution in a single molecule.
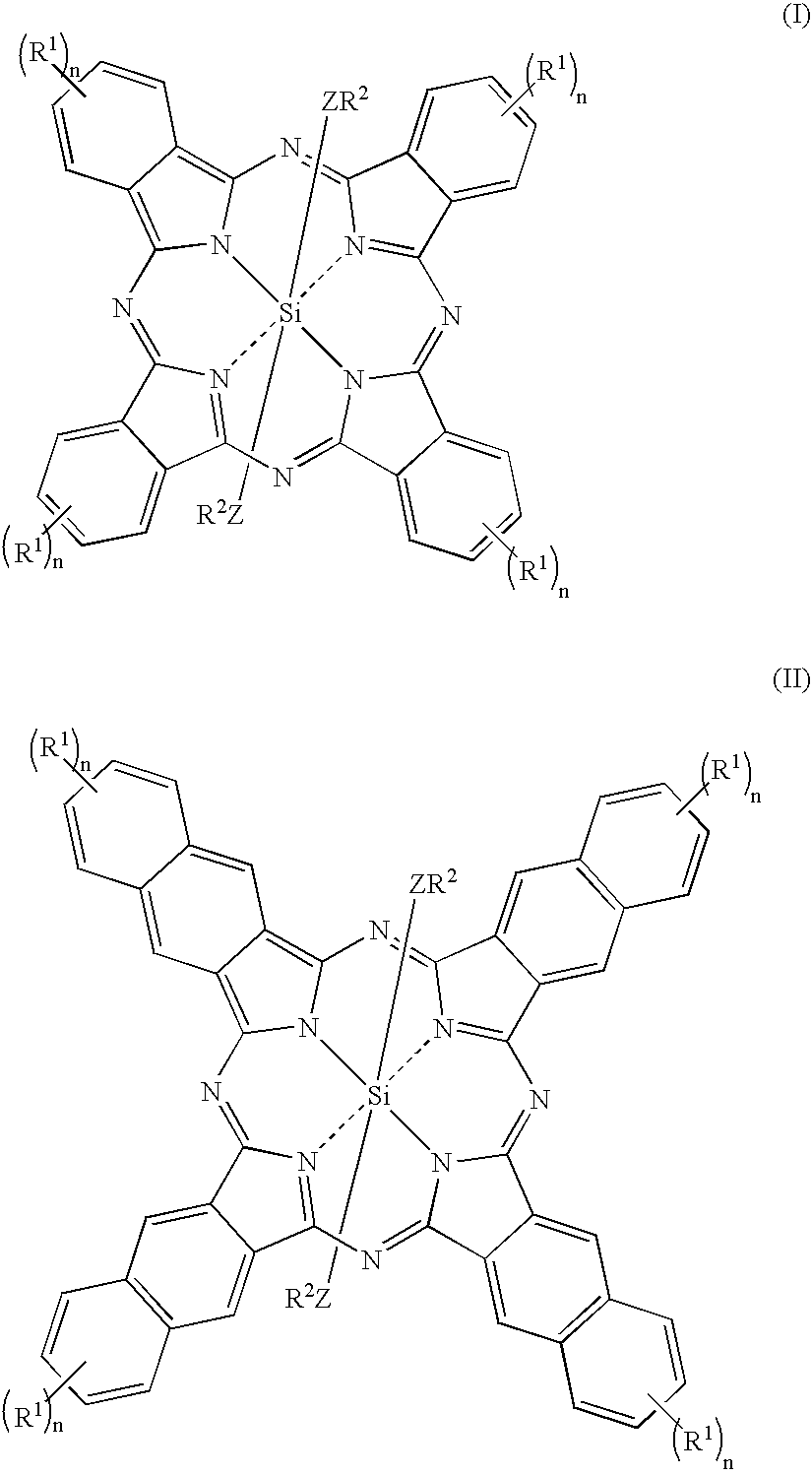
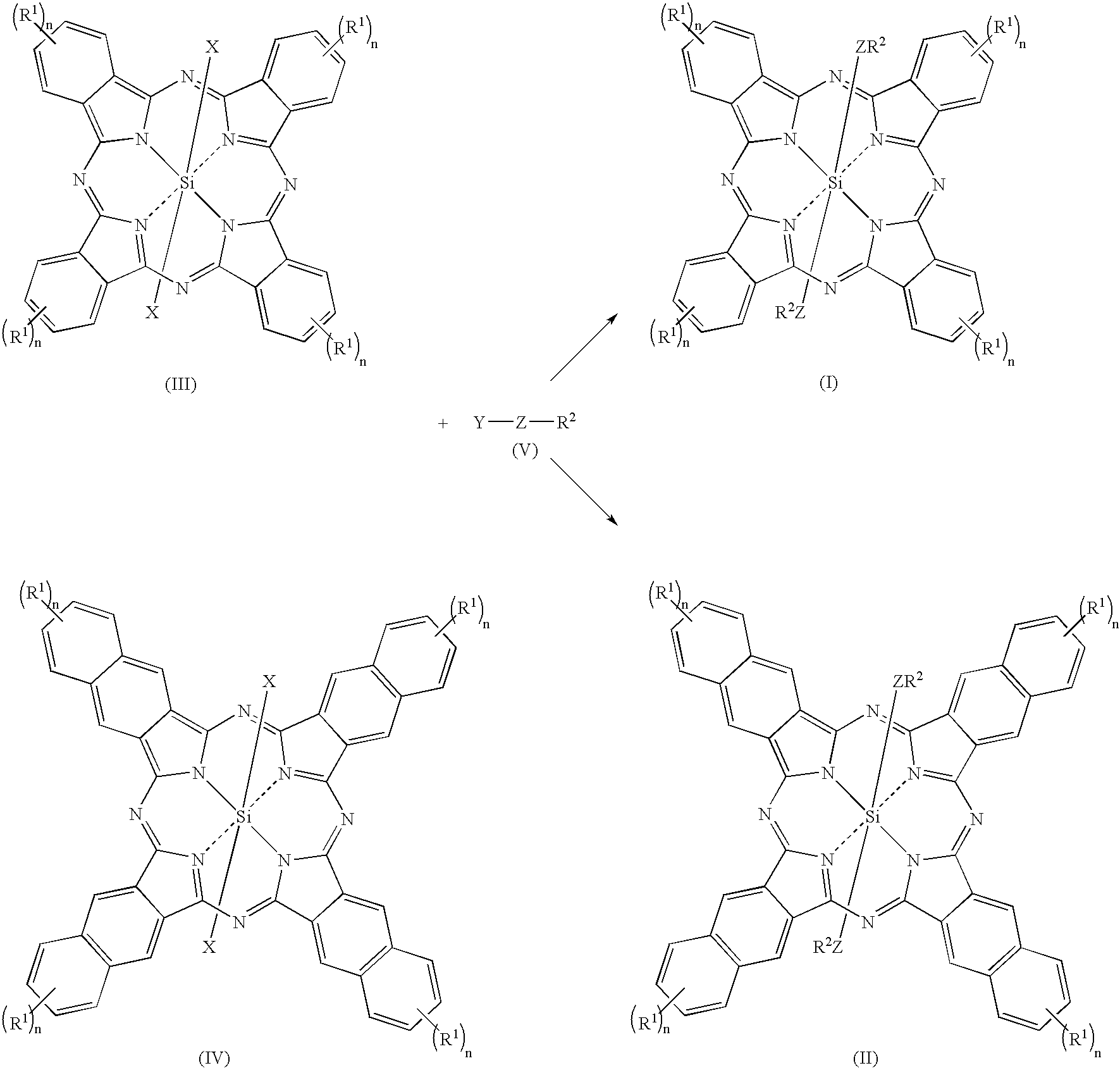
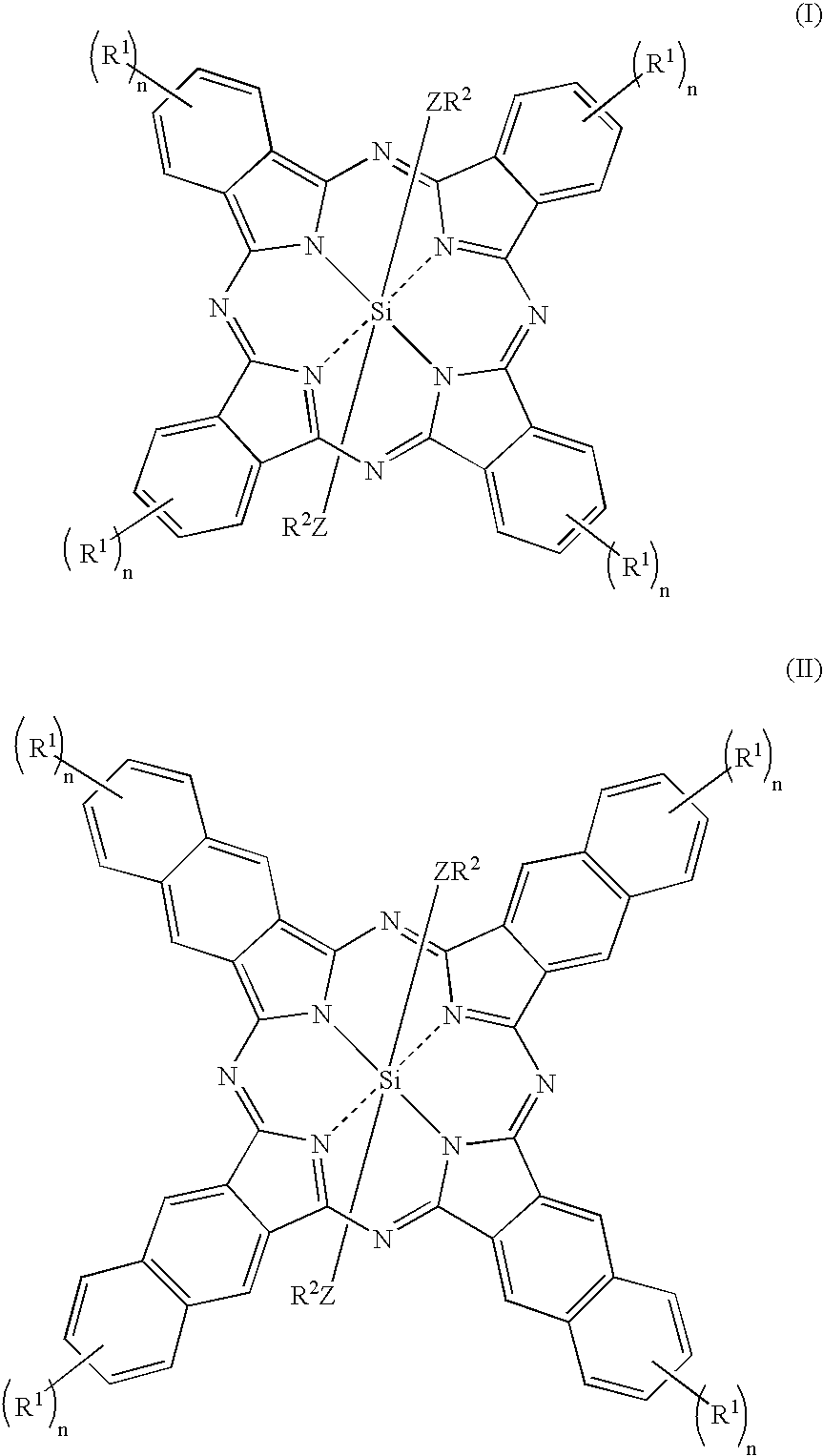
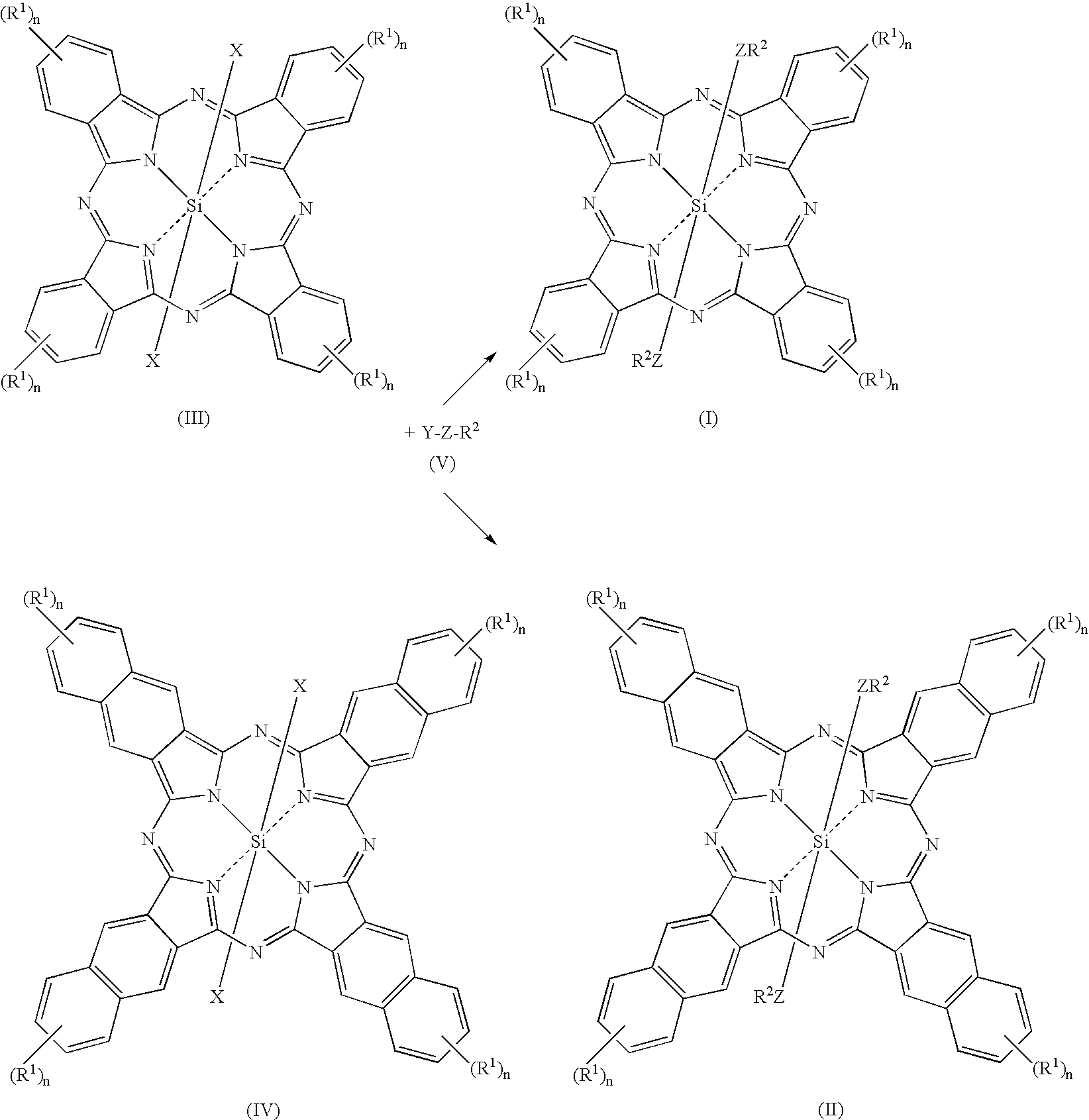
Novel Fluorinated silicon (IV) phthalocyanines and naphthalocyanines for electrophoretic, magnetophoretic or electromagnetophoretic display
This invention relates to stable colorants of high extinction coefficient and high solubility or dispersibility for an electrophoretic, magnetophoretic or electromagnetophoretic display. More particularly, it relates to stable colorants for a microcup-based electrophoretic or electromagnetophoretic display the cells of which are filled with charged and / or magnetic particles dispersed in a halogenated, preferably a fluorinated, solvent. The use of the stable colorants allows the display to be of superior contrast ratio and longevity, and suitable for high-quality imagery applications.
Owner:SIPIX IMAGING INC
Synthetically expedient water-dispersible IR dyes
Owner:BASF SE
Ink formulations comprising gallium naphthalocyanines
There is provided an aqueous formulation comprising an IR-absorbing naphthalocyanine dye of formula (II):or a salt form thereof, wherein:M is Ga(A1);A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4 or —O(CH2CH2O)eRe wherein e is an integer from 2 to 10 and Re is H, C1-8 alkyl or C(O)C1-8 alkyl;R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy;R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz);R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl; andRx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy;The formulation has a pH in the range of 3.5 to 7 and is particularly suitable for use as an IR-absorbing inkjet ink, providing compatibility with known CMYK inks together with an optimally red-shifted λmax.
Owner:BASF AG
Phthalocyanine dyes suitable for use in offset inks
InactiveUS7559983B2Improve visibilityLess absorptionOrganic chemistryReactive dyesSulfonatePhosphonium
Owner:BASF SE
Gallium naphthalocyanine salts for use as improved infrared dyes
There is provided an IR-absorbing naphthalocyanine dye of formula (I):whereinM is Ga(A1);A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4 or —O(CH2CH2O)eRe wherein e is an integer from 2 to 10 and Re is H, C1-8 alkyl or C(O)C1-8 alkyl;R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy;R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz);R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl;Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; andeach B is independently selected from a base, wherein BH+ has a pKa of between 4 and 9. The dye is suitable for use in IR-absorbing inkjet inks, providing compatibility with known CMYK inks together with an optimally red-shifted λmax.
Owner:BASF SE
Ink formulations comprising gallium naphthalocyanines
There is provided an aqueous formulation comprising an IR-absorbing naphthalocyanine dye of formula (II):or a salt form thereof, wherein:M is Ga(A1);A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4 or —O(CH2CH2O)eRe wherein e is an integer from 2 to 10 and Re is H, C1-8 alkyl or C(O)C1-8 alkyl;R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy;R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz);R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl; andRx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy;The formulation has a pH in the range of 3.5 to 7 and is particularly suitable for use as an IR-absorbing inkjet ink, providing compatibility with known CMYK inks together with an optimally red-shifted λmax.
Owner:BASF SE
Flame retardant compositions for flammable plastics and flame retarded plastic compositions containing the same
A flame retardant composition for rendering flammable plastics flame retardant is provided. The composition comprises a brominated flame retardant, a free-radical generator selected from 2,3-dimethyl-2,3-diphenylbutane or its homologs, and a phthalocyanine or naphthalocyanine complex with a metal of groups 7 to group 10 of the IUPAC periodic chart. The quantity of the brominated flame retardant may be saved when incorporating into flammable plastic materials in conjunct with the free-radical generator and the phathalocyanine or naphthalocyanine complex of the above type.
Owner:DAI ICHI KOGYO SEIYAKU CO LTD
Solar control laminates
ActiveUS20070228340A1Reduce transmissionPhotosensitive materialsSynthetic resin layered productsIonomerControl layer
Provided is a solar control composition comprising an infrared absorbing phthalocyanine, naphthalocyanine or rylene compound and an ethylene acid copolymer or an ionomer of an ethylene acid copolymer. Further provided are solar control laminates comprising the solar control composition of the invention.
Owner:DOW GLOBAL TECH LLC
Gallium naphthalocyanine salts for use as improved infrared dyes
There is provided an IR-absorbing naphthalocyanine dye of formula (I):whereinM is Ga(A1);A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4 or —O(CH2CH2O)eRe wherein e is an integer from 2 to 10 and Re is H, C1-8 alkyl or C(O)C1-8 alkyl;R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy;R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz);R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl;Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; andeach B is independently selected from a base, wherein BH+ has a pKa of between 4 and 9.The dye is particularly suitable for use in IR-absorbing inkjet inks, providing compatibility with known CMYK inks together with an optimally red-shifted λmax.
Owner:BASF AG
Synthetically expedient water-dispersible IR dyes having improved lightfastness
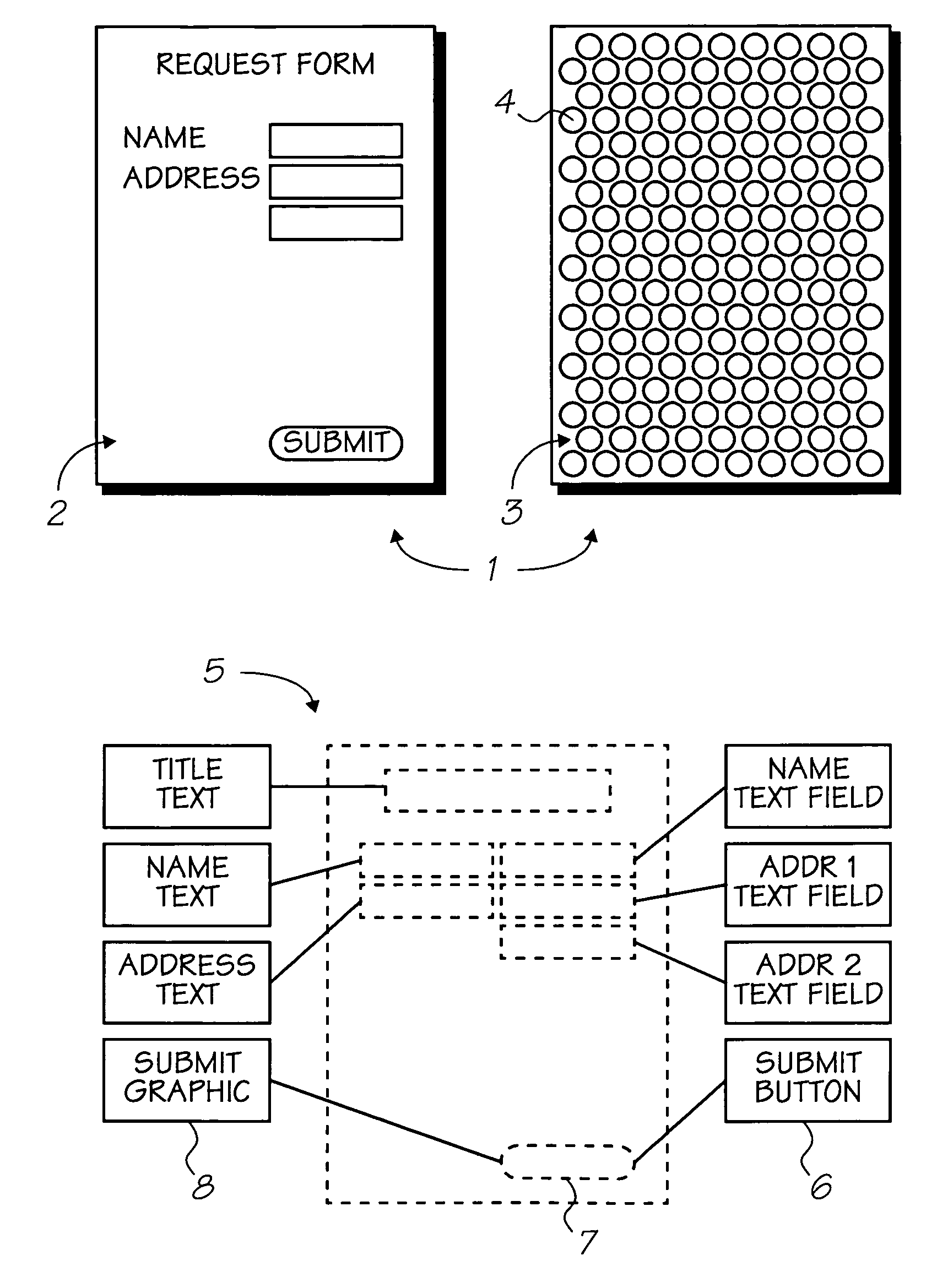
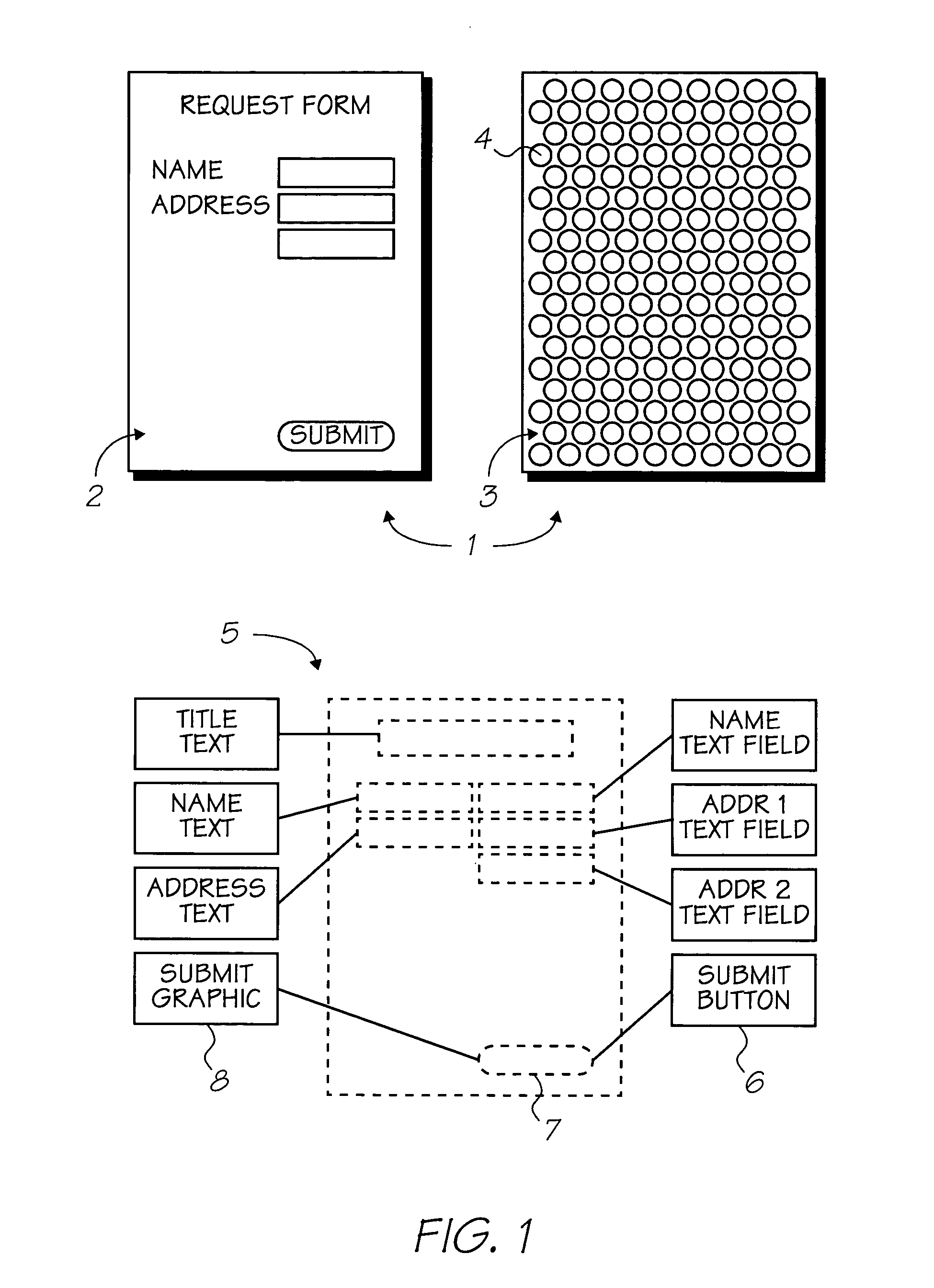
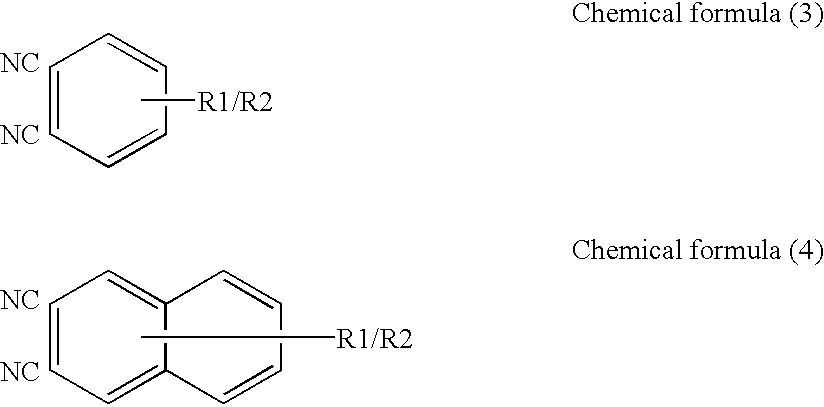
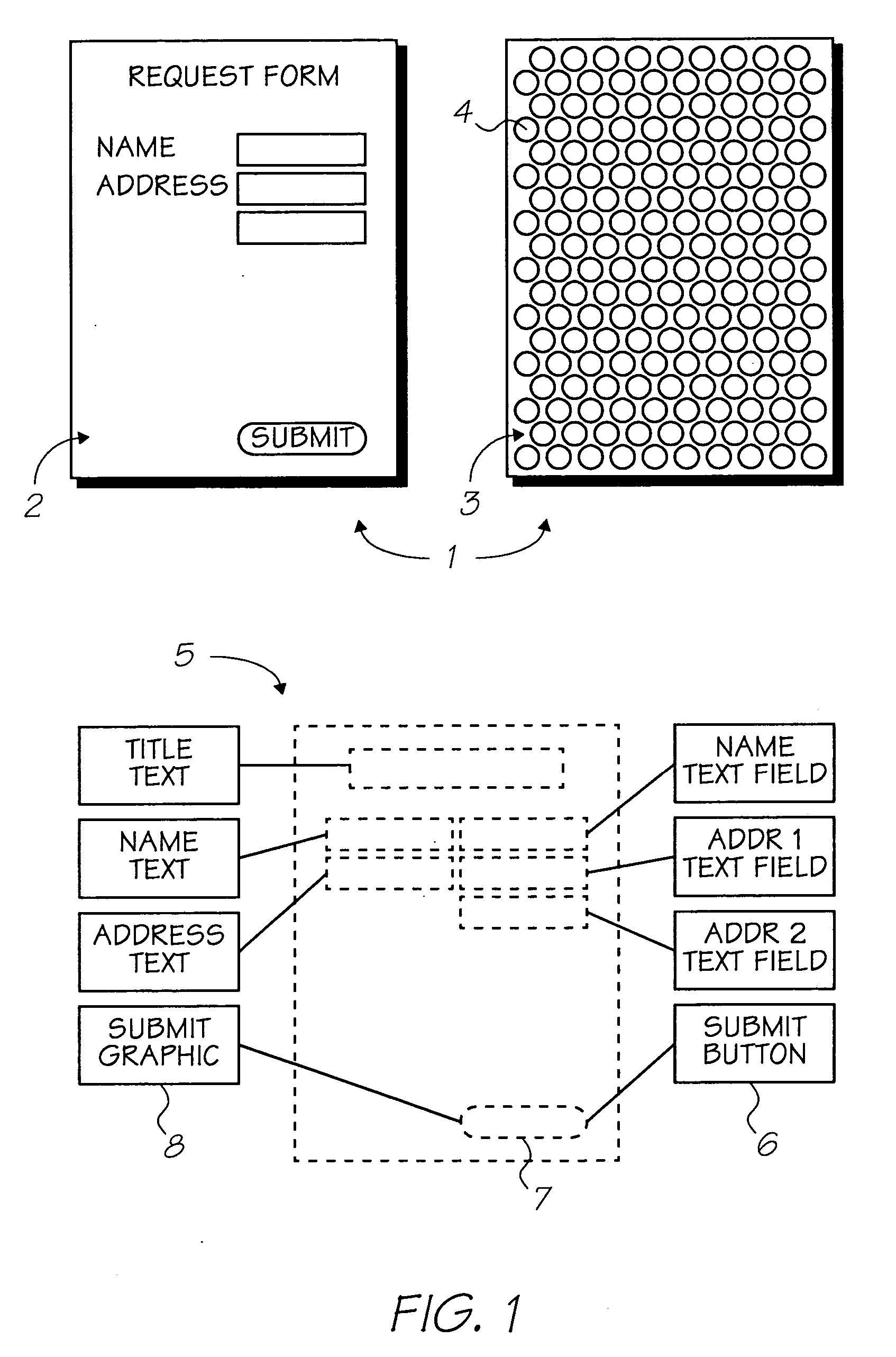
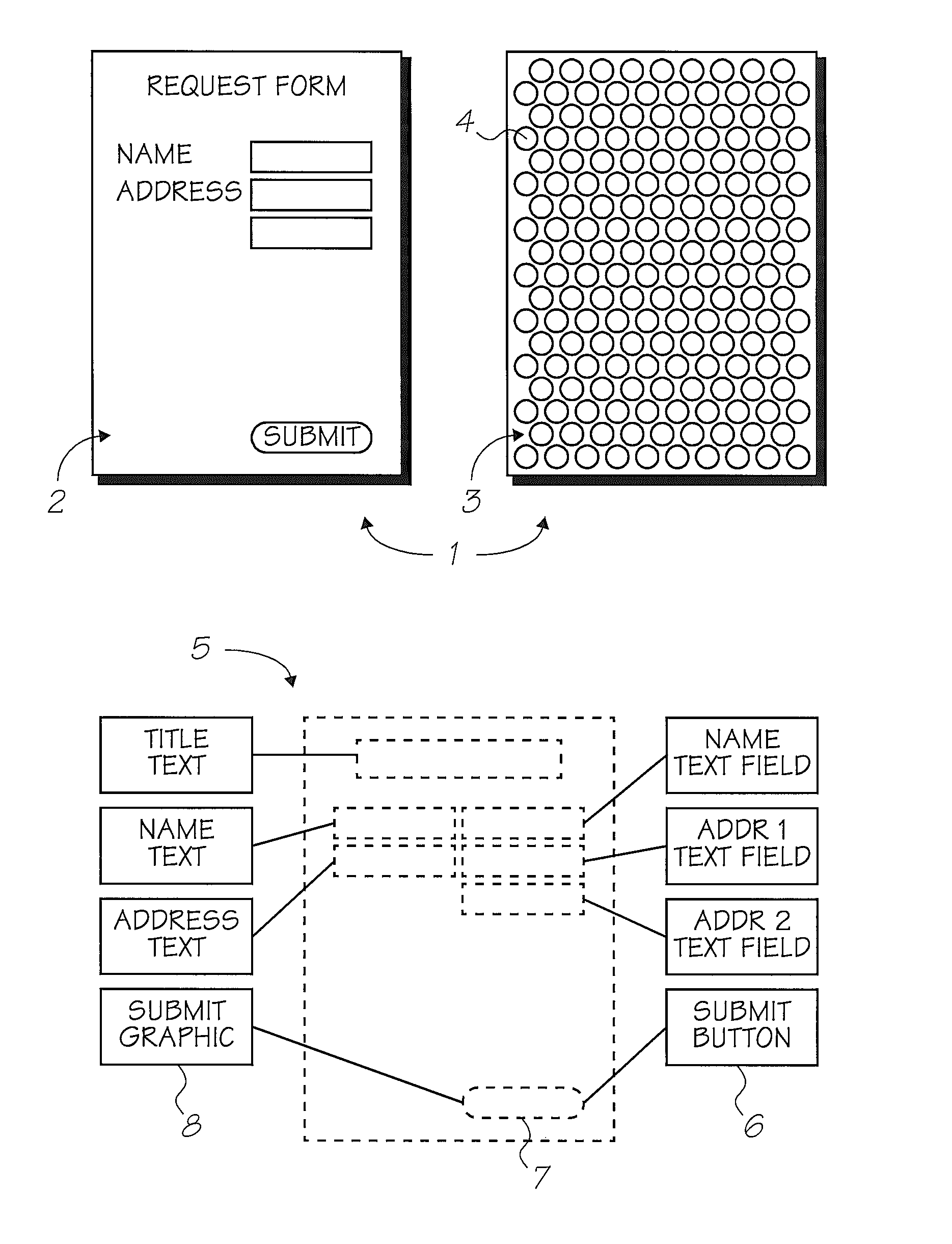
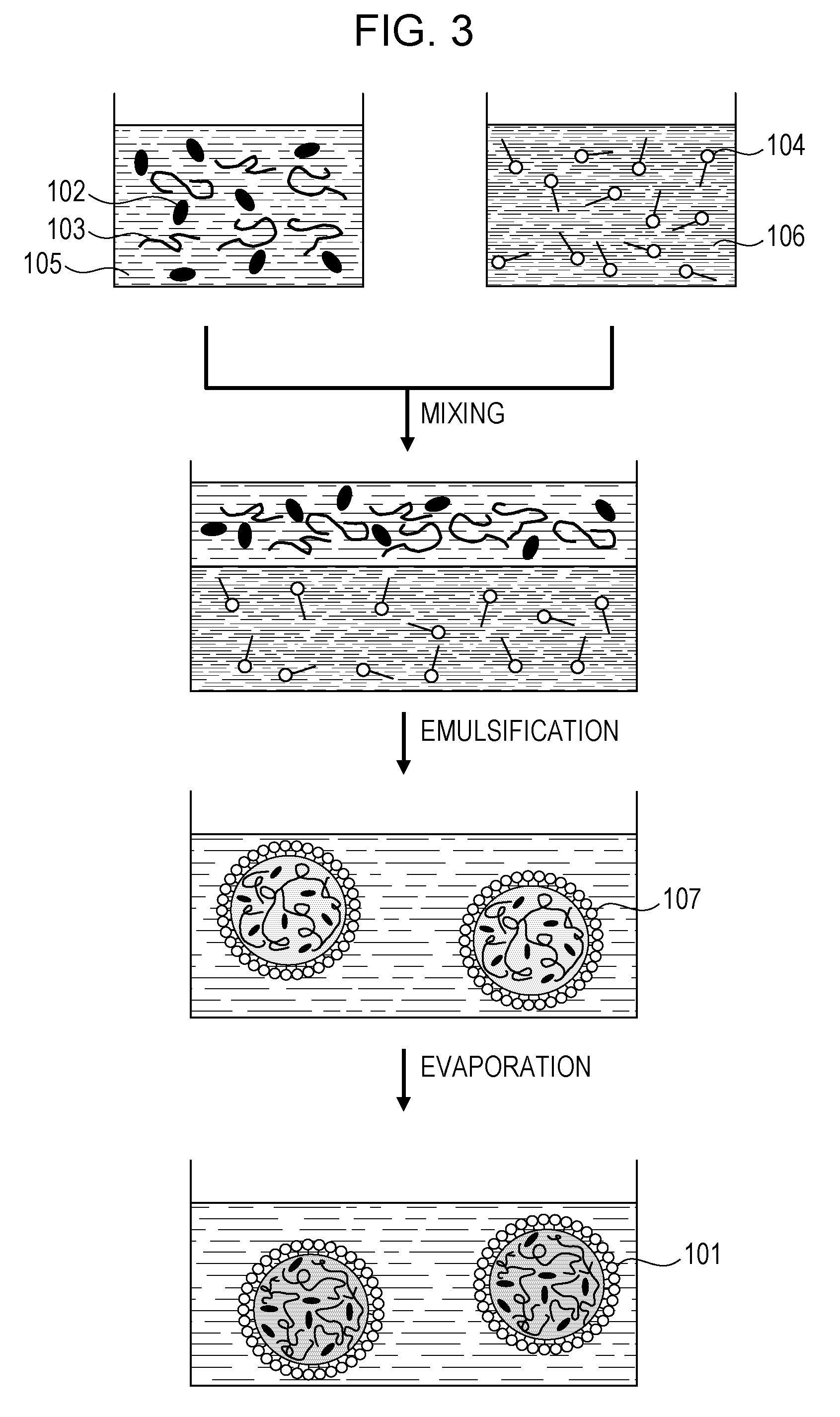
The present invention provides an IR-absorbing naphthalocyanine dye of formula (I): wherein M is selected from Ga(A1); A1 is an axial ligand selected from —OH, halogen (preferably Cl), —OR3, a hydrophilic ligand and / or a ligand suitable for reducing cofacial interactions; R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy (preferably C1-6 alkoxy); R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz); and Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; W is a hydrophilic group; n1 is 0, 1, 2 or 3; n2 is 0, 1, 2 or 3; n3 is 0, 1, 2 or 3; n4 is 0, 1, 2 or 3; provided that at least one of n1, n2, n3 or n4 is greater than 0. Dyes of this type are especially suitable for use in netpage and Hyperlabel™ systems.
Owner:BASF AG
Method of preparing naphthalocyanines
InactiveUS7671194B2Easy to synthesizeImprove scalabilityInksPorphines/azaporphinesNaphthalocyanineChemistry
A method of preparing a naphthalocyanine is provided. The method comprises the steps of: (i) providing a tetrahydronaphthalic anhydride; (ii) converting said tetrahydronaphthalic anhydride to a benzisoindolenine; and (iii) macrocyclizing said benzisoindolenine to form a naphthalocyanine.
Owner:BASF AG
Toner for optical fixing and image formation apparatus using it
It is a toner for optical fixing which contains a binder resin, a colorant, and an infrared light absorbent, and the infrared light absorbent has a coloring opacity of 20 or less, and of phthalocyanine compound and / or naphthalocyanine compound.
Owner:FUJITSU LTD
Inkjet lnks for printing coded data comprising naphthalocyanine dyes
The present invention provides an inkjet ink comprising an IR-absorbing naphthalocyanine dye of formula (I): wherein M is selected from Si(A1)(A2), Ge(A1)(A2), Ga(A1), Mg, Al(A1), TiO, Ti(A1)(A2), ZrO, Zr(A1)(A2), VO, V(A1)(A2), Mn, Mn(A1), Fe, Fe(A1), Co, Ni, Cu, Zn, Sn, Sn(A1)(A2), Pb, Pb(A1)(A2), Pd and Pt; A1 and A2 are axial ligands, which may be the same or different, and are selected from —OH, halogen or —OR3; R1 and R2 are selected from H or C1-12 alkoxy; R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz); and Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; W is selected from a sulfonic acid group (including salts thereof) or a sulfonamide group; n1 is 0, 1, 2 or 3; n2 is 0, 1, 2 or 3; n3 is 0, 1, 2 or 3; n4 is 0, 1, 2 or 3; provided that at least one of n1, n2, n3 or n4 is greater than 0. Inks of this type are especially suitable for use in netpage and Hyperlabel systems.
Owner:BASF SE
Inkjet ink comprising gallium naphthalocyanine dye
There is provided an inkjet ink comprising an IR-absorbing dye of formula (I): wherein M is Ga(A1); A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4, a hydrophilic ligand and / or a ligand suitable for reducing cofacial interactions; R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy; R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz); and R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; W is a hydrophilic group, each W group being independently selected from a substituent comprising a sulfonic acid group (including salts thereof), or a substituent comprising a sulfonamide group; n1 is 0, 1, 2 or 3; n2 is 0, 2, or 3; n3 is 0, 1, 2 or 3; n4 is 0, 1, 2 or 3; provided that at least one of n1, n2, n3 or n4 is greater than 0. Such inks are suitable for use in netpage, Hyperlabel and other applications.
Owner:BASF AG
Ink comprising gallium naphthalocyanine dye
An ink comprising an IR-absorbing naphthalocyanine dye of formula (I) is described: Wherein M is Ga(A1); A1 is an axial ligand selected from —OH, halogen, —OR3, —OC(O)R4; R1 and R2 may be the same or different and are selected from hydrogen or C1-12 alkoxy; R3 is selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl or Si(Rx)(Ry)(Rz); and R4 is selected from C1-12 alkyl, C5-12 aryl or C5-12 arylalkyl Rx, Ry and Rz may be the same or different and are selected from C1-12 alkyl, C5-12 aryl, C5-12 arylalkyl, C1-12 alkoxy, C5-12 aryloxy or C5-12 arylalkoxy; and W is a sulfonic acid group, including salts thereof.
Owner:BASF AG
Solar control laminates
InactiveUS20070228341A1Reduce transmissionLiquid surface applicatorsSynthetic resin layered productsControl layerNaphthalocyanine
Provided is a solar control composition comprising an infrared absorbing phthalocyanine compound or naphthalocyanine compound and a resin having a modulus from 20,000 psi (138 MPa) to 1000 psi (7 MPa) and solar control laminates comprising the solar control composition of the invention.
Owner:EI DU PONT DE NEMOURS & CO
Naphthalocyanine dye and ink containing the same
InactiveUS20120139994A1High extinctionRadiation pyrometryOrganic chemistryNaphthalocyaninePhotochemistry
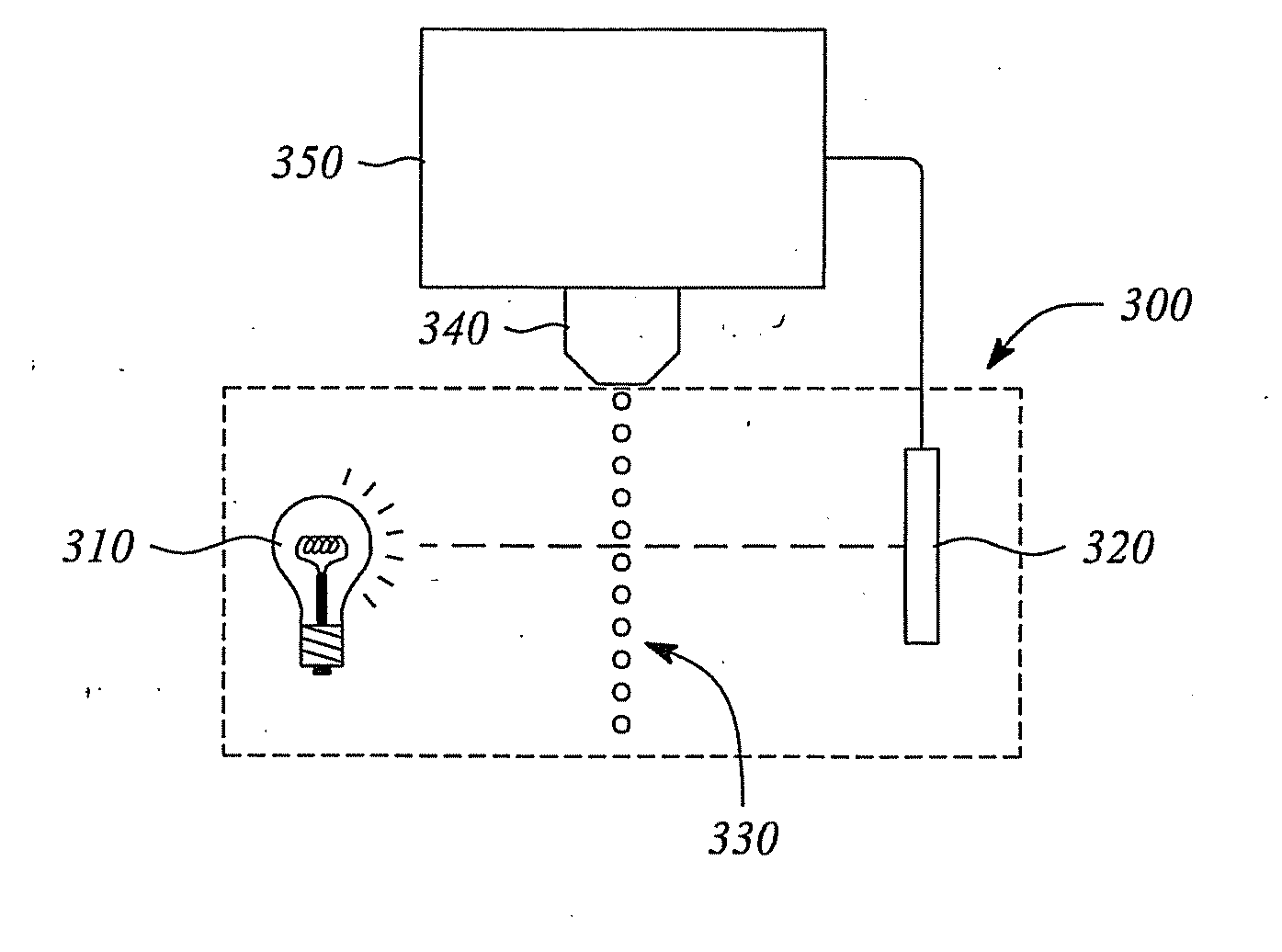
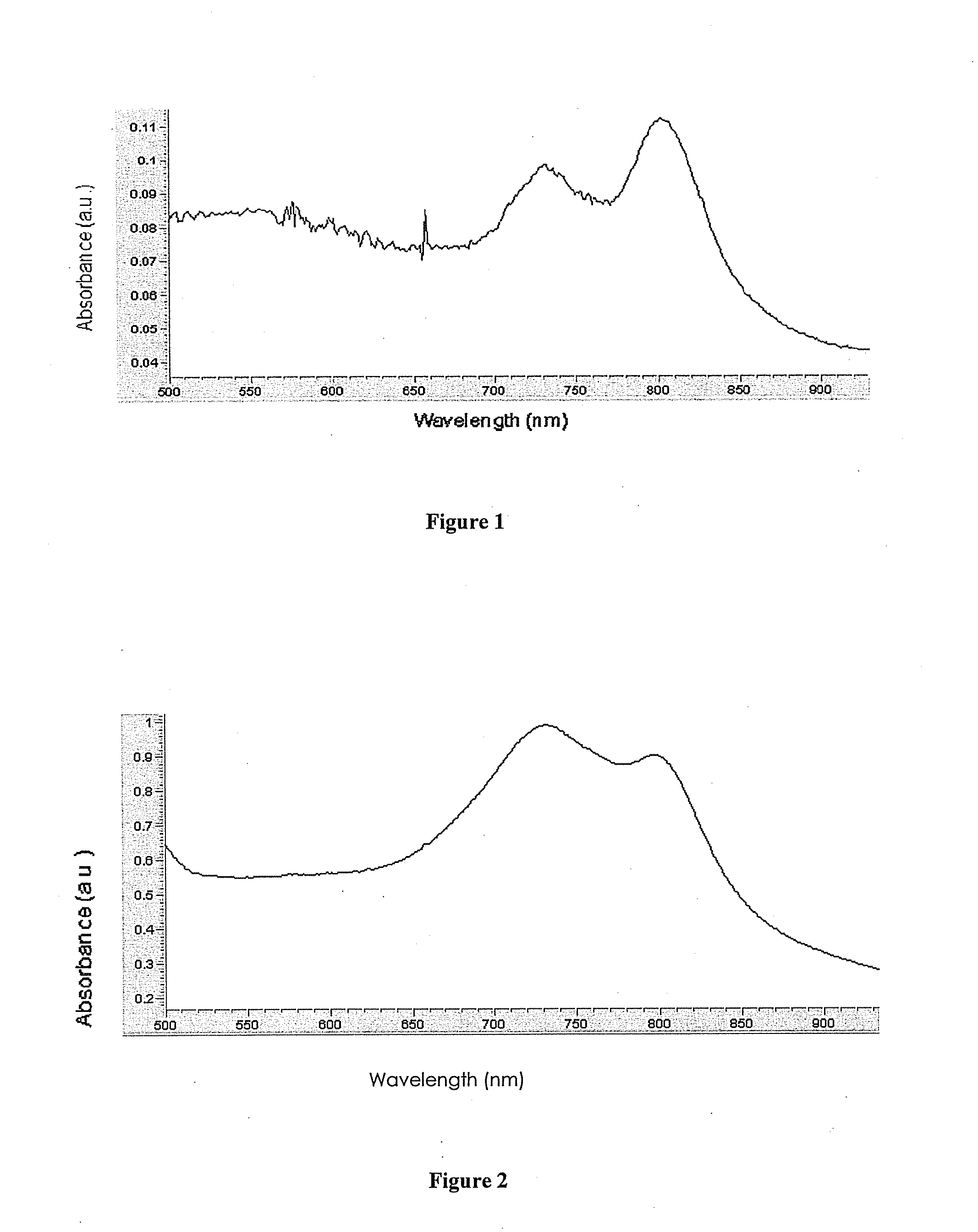
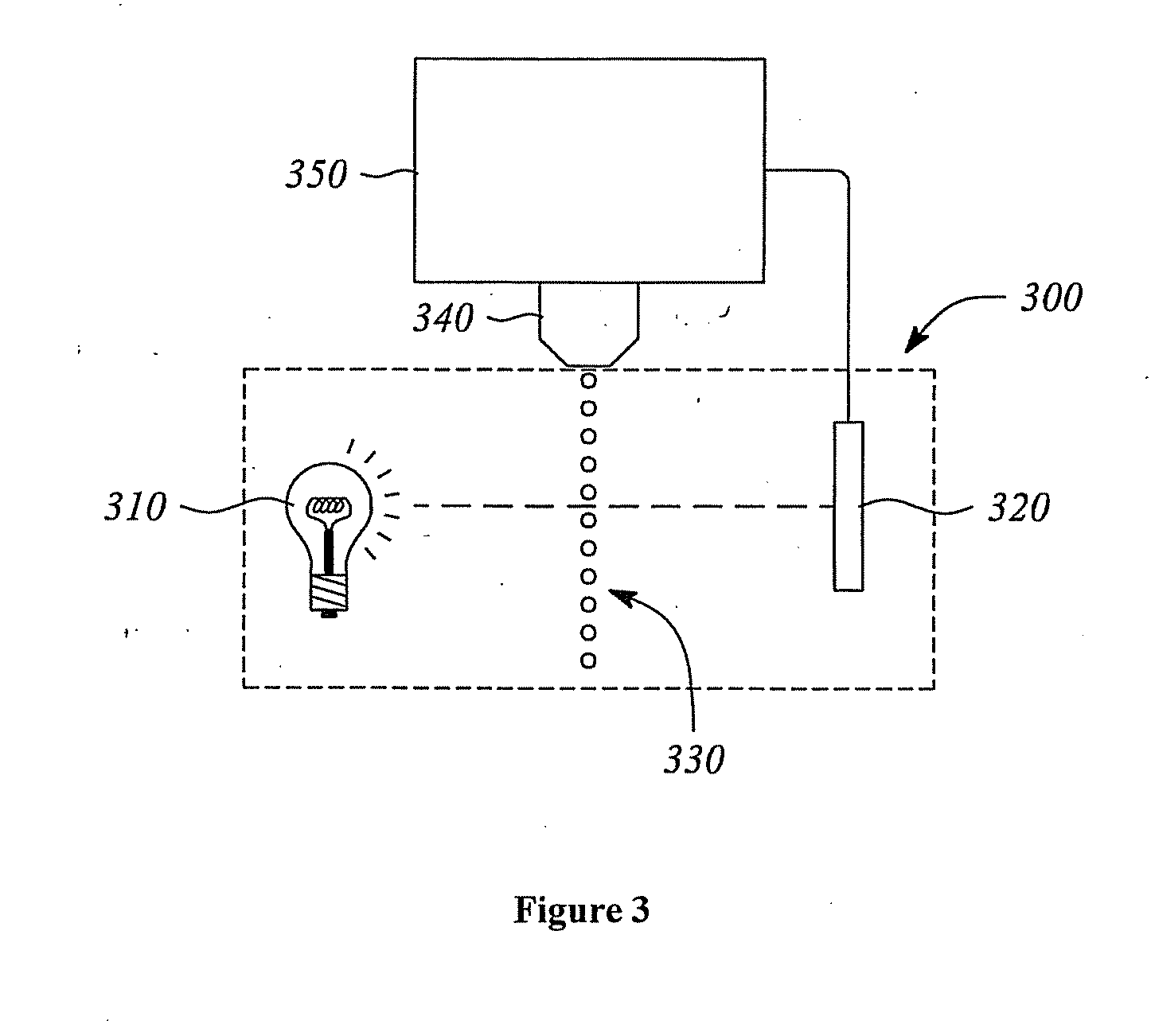
The present disclosure includes naphthalocyanine dyes or fused naphthalocyanine dyes represented by one of the general structures I to XV; inkjet ink formulations including the naphthalocyanine dyes; and a detection system (300) including the dyes.
Owner:HEWLETT PACKARD DEV CO LP
Organic semiconducting material, and film, organic electronic device and infrared dye composition each including said material
ActiveUS20090054641A1Improve stabilityFinal product manufactureReactive dyesHydrogen atomSemiconductor materials
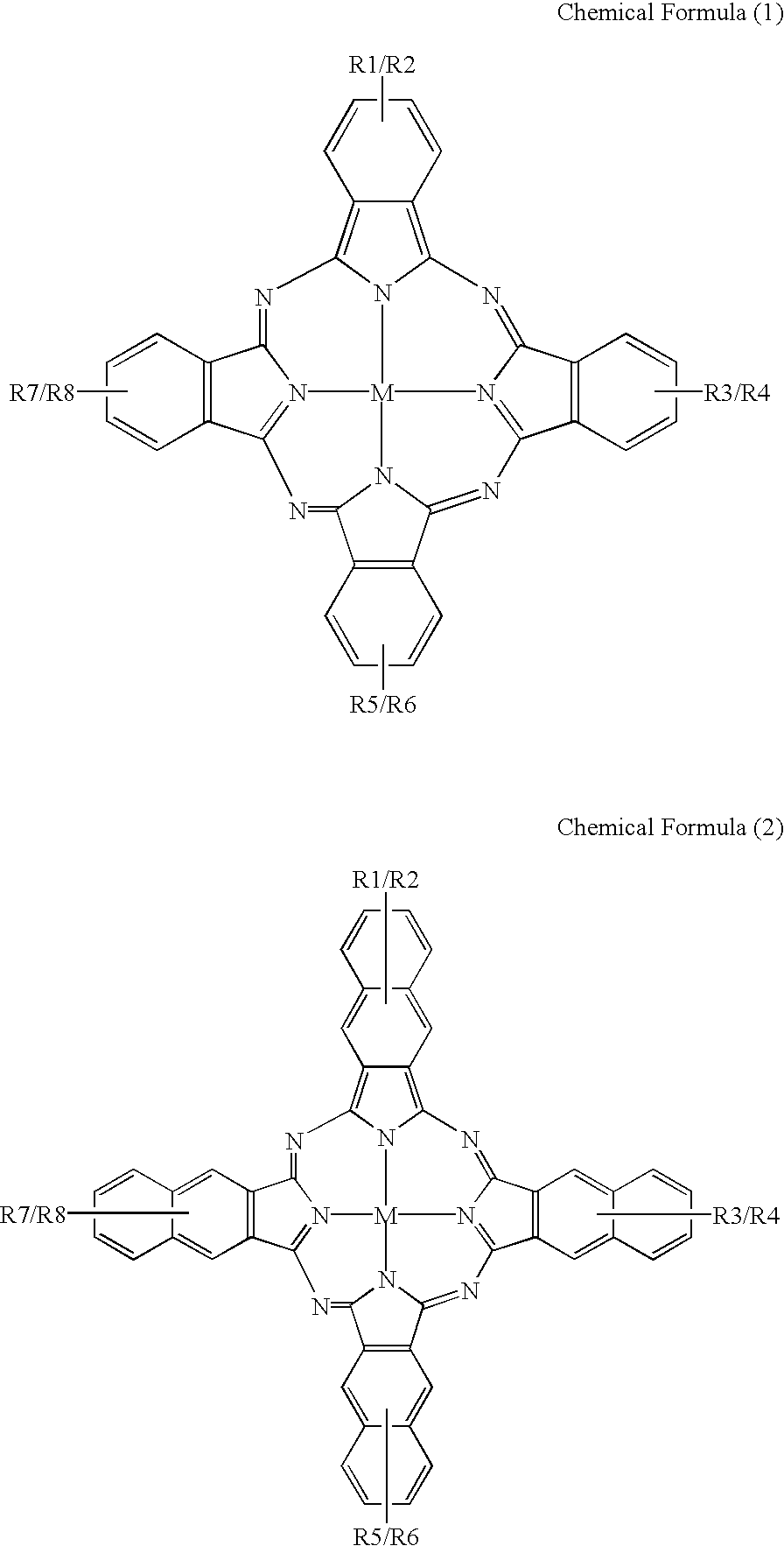
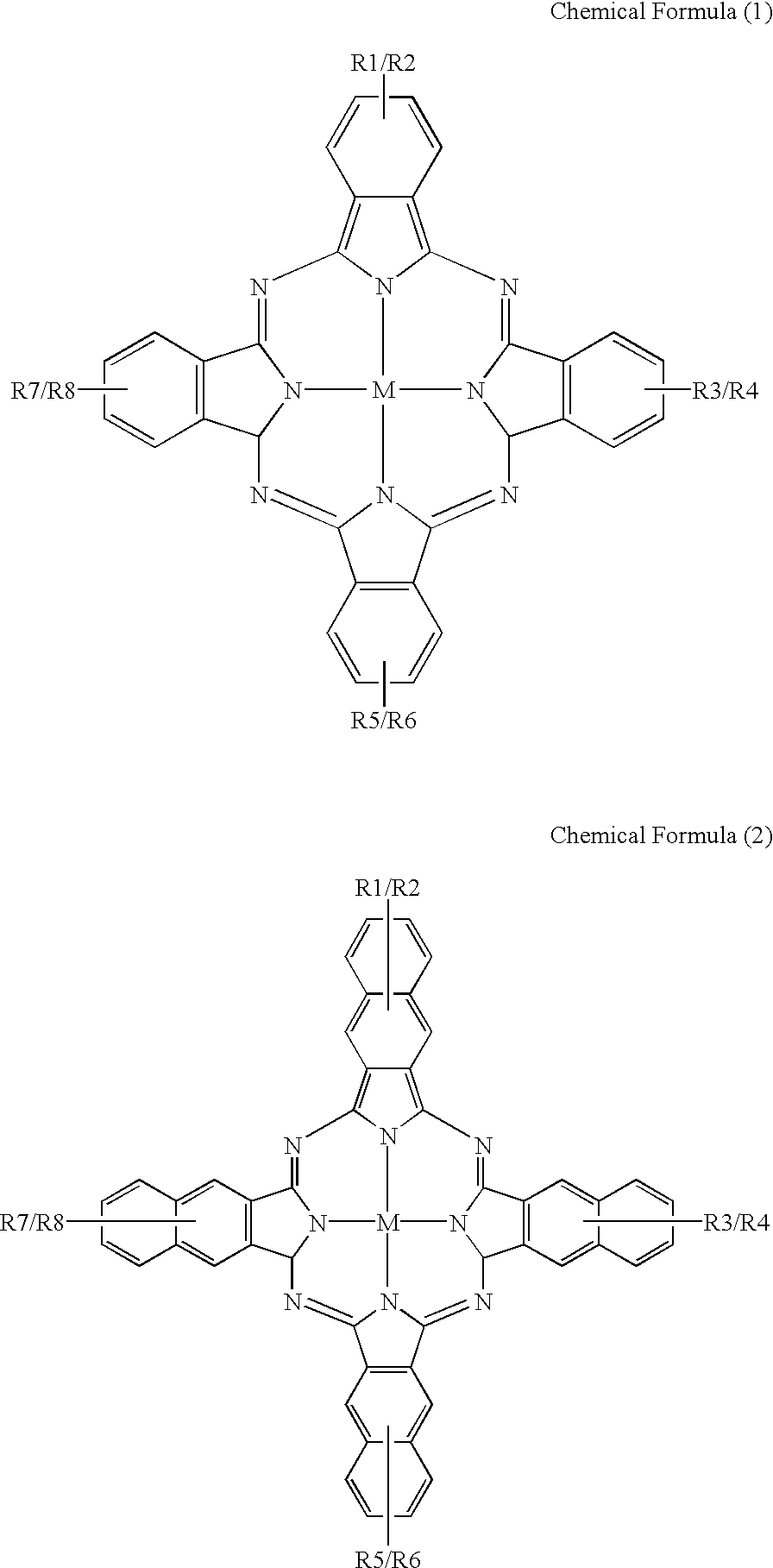
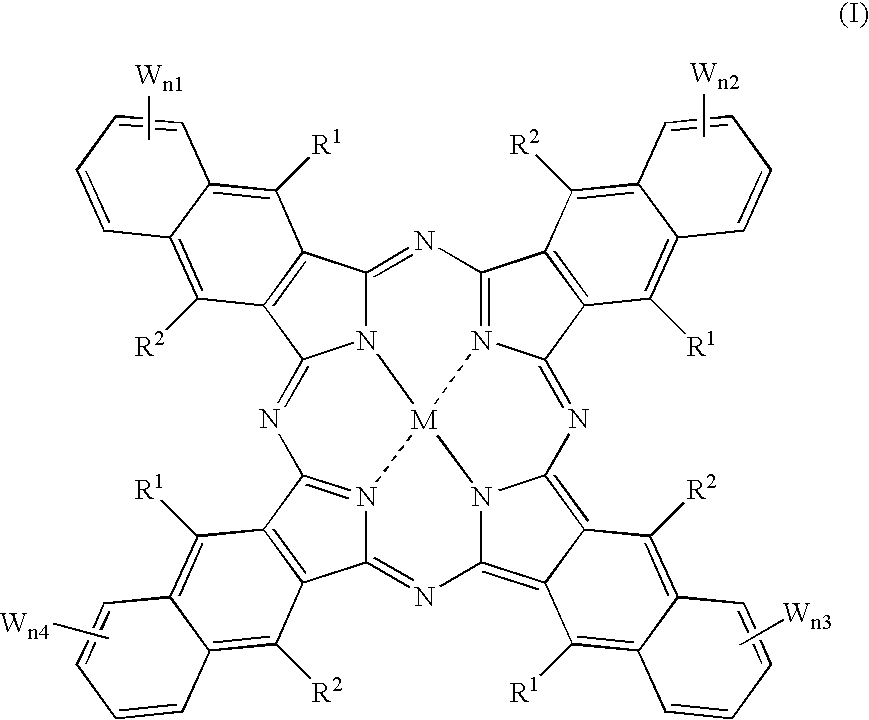
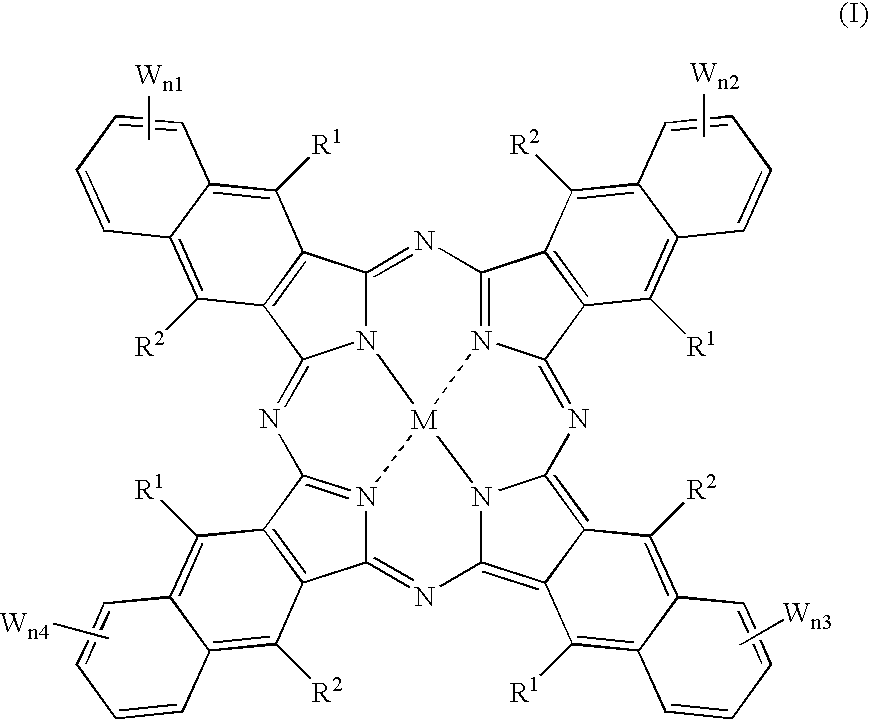
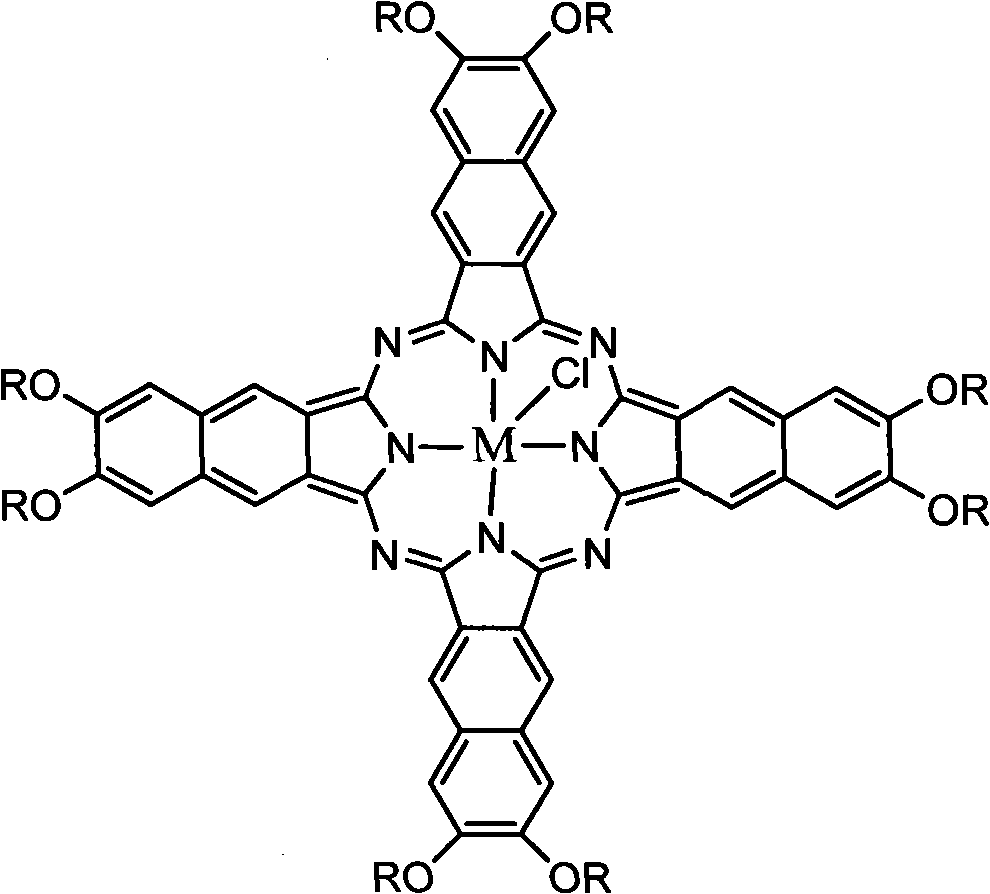
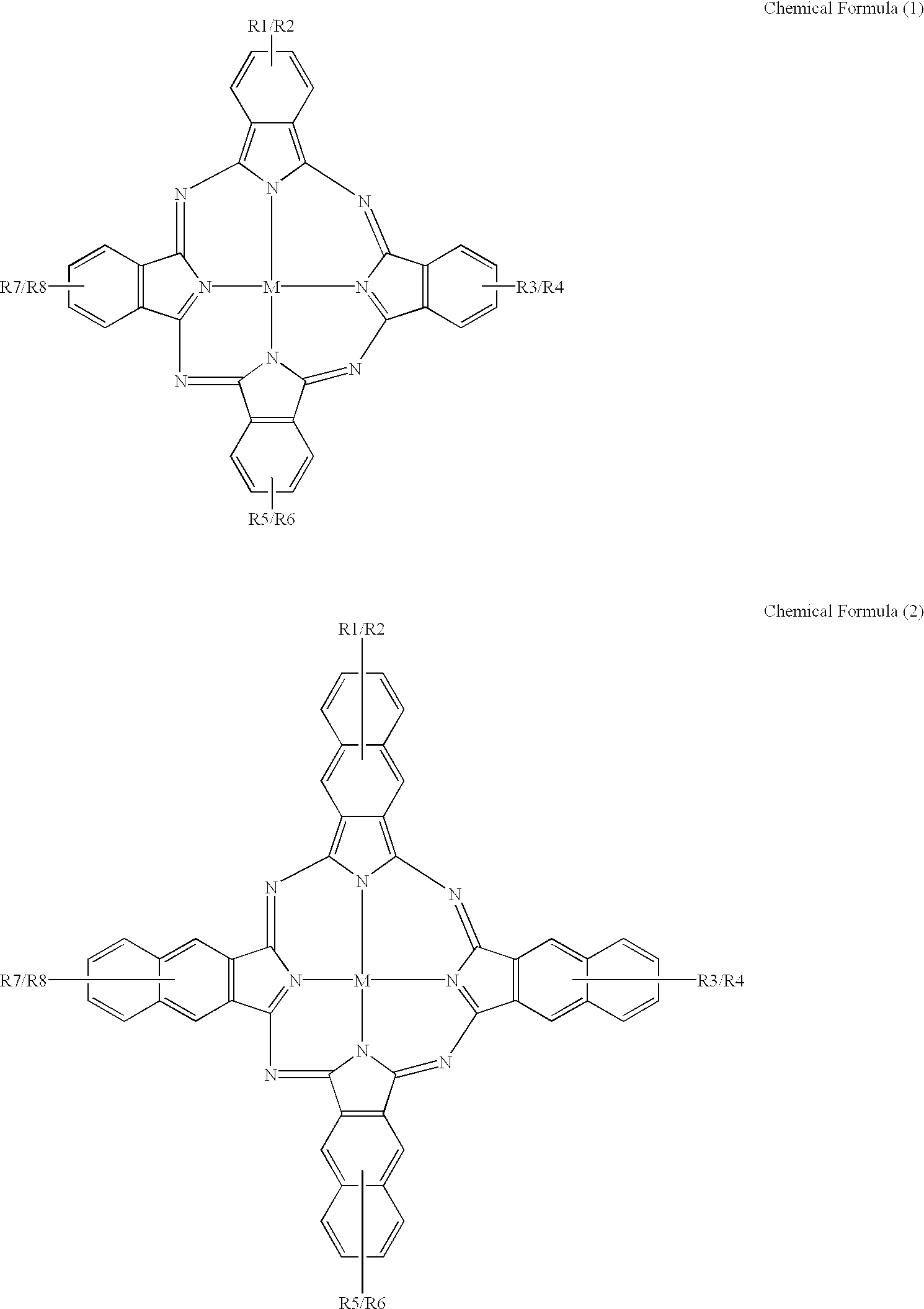
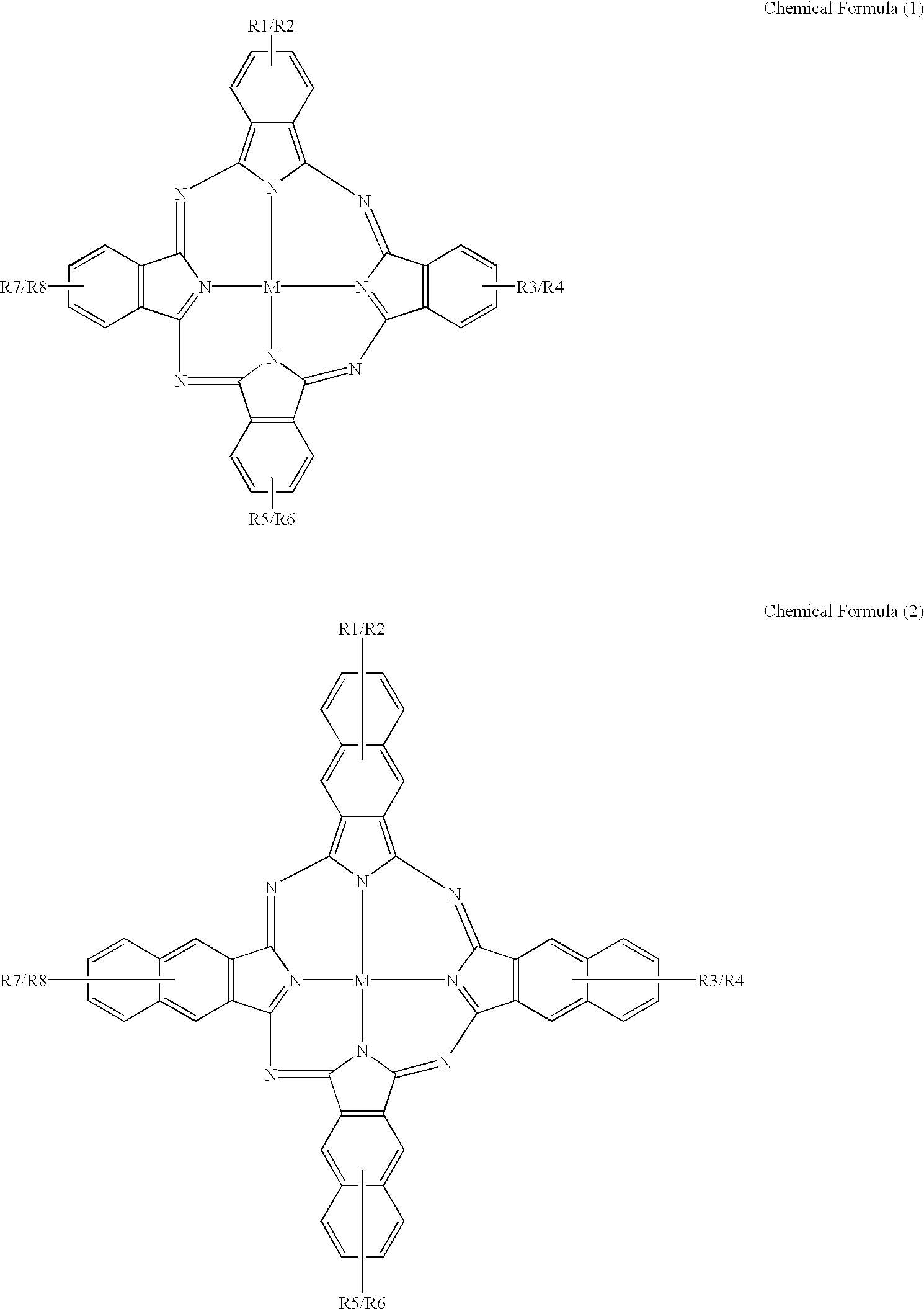
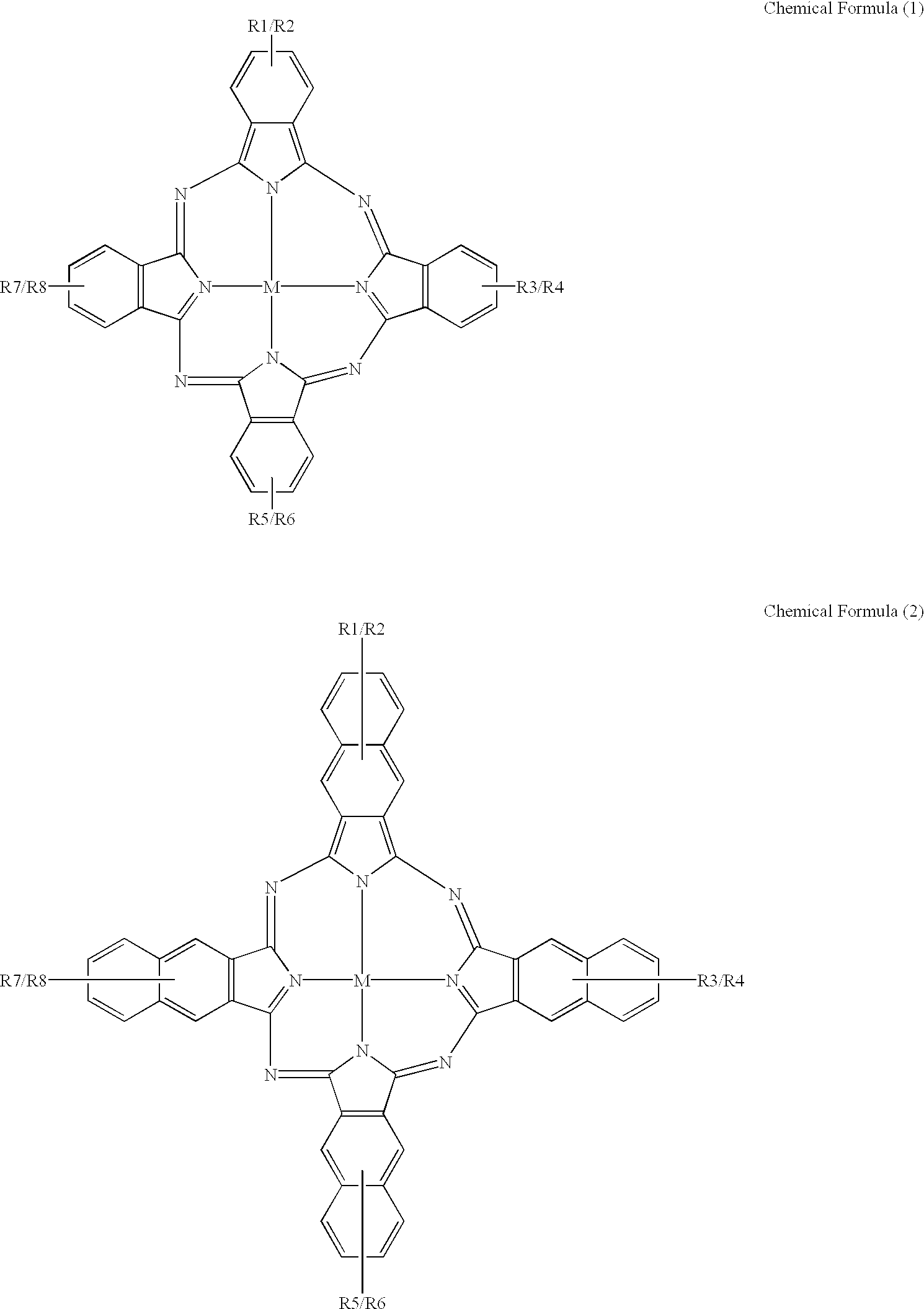
An organic semiconducting material comprises a naphthalocyanine derivative represented by formula (1);wherein M represents Si, Ge or Sn, R1 to R3 represent substituents other than a hydrogen atom except that all of R1 to R3 are identical straight-chain alkyl groups, and R4 to R27 each independently represents a hydrogen atom or a substituent.
Owner:FUJIFILM CORP
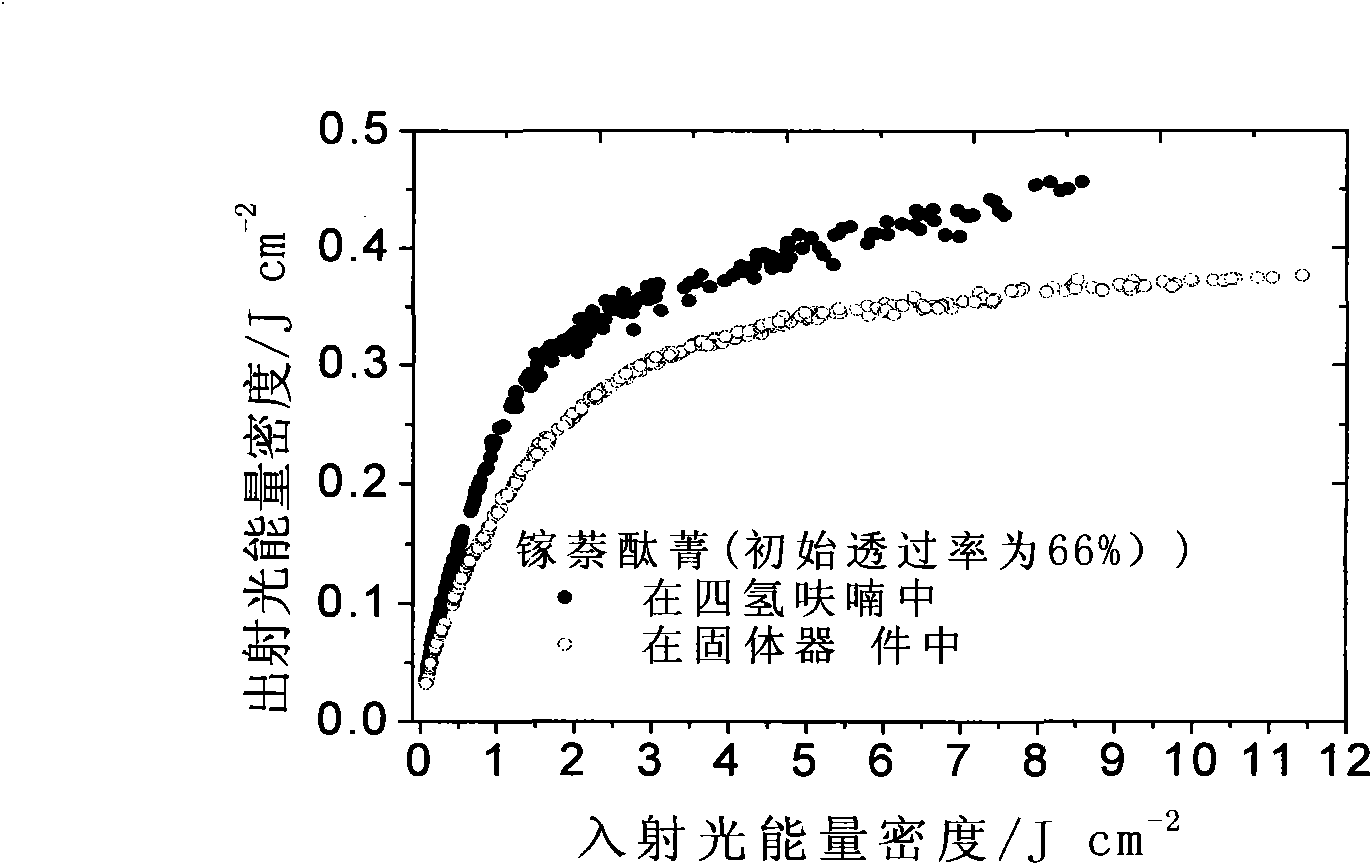
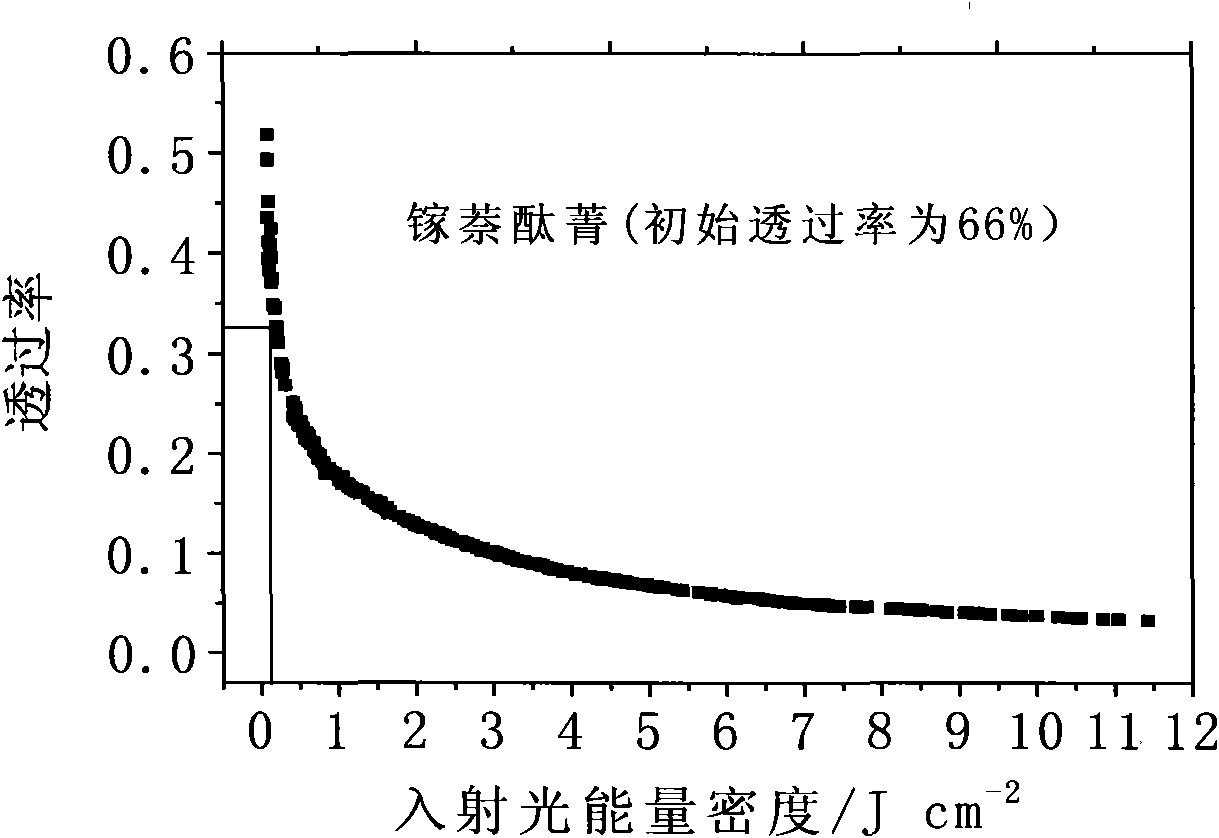
Solid naphthalocyanine device with optical limiting properties
ActiveCN101561614AOvercoming fragileOvercome the defect of poor laser resistanceNon-linear opticsInorganic compoundElectron
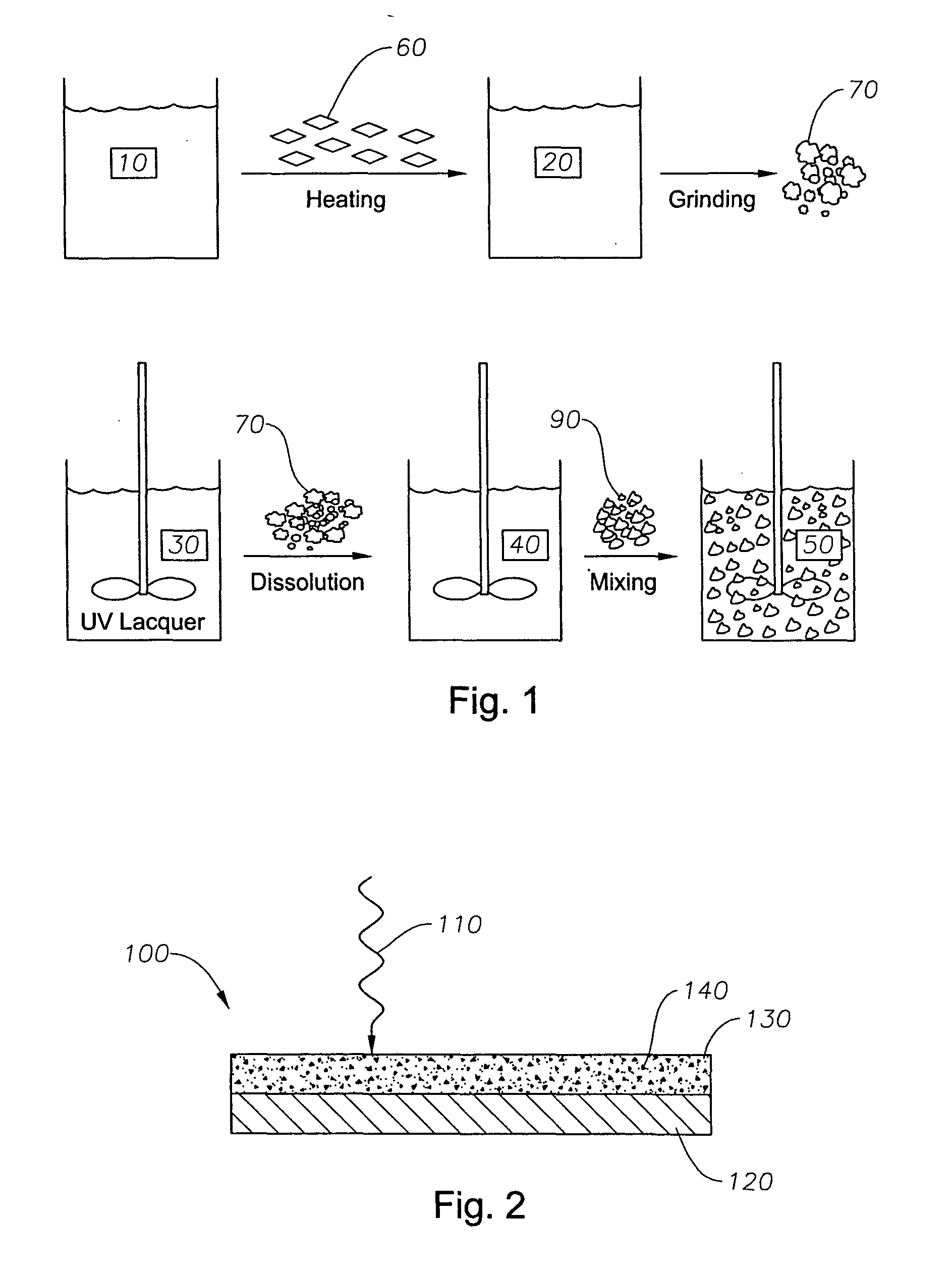
The invention relates to a solid naphthalocyanine device with excellent optical limiting properties. The device is made by the following steps: adding tetraethoxysilane, diglycidyl ether propyltrimethoxy siloxane, analytically pure ethanol and acidic aqueous solution to a reactor; stirring in an enclosed reactor for 2-3h, then opening the reactor and continuing stirring for 1-2h; adding a naphthalocyanine solution and stirring to cause the mixture to become viscous; transferring the viscous mixture to a mould, and taking out after drying to obtain the novel solid naphthalocyanine device with optical limiting properties. The device is made by an organosilicon modification method combined with doped inorganic salt, and by doping naphthalocyanine with a larger pi electron conjugated system to form a continuous random network system with bonded chemical bonds of organic components and inorganic components instead of physical hybrid. The device has good laser resistance and excellent optical limiting properties.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Toner for optical fixing, manufacturing method therefor and image formation apparatus using it
It is a toner for optical fixing which contains a binder resin, a colorant, and an infrared light absorbent, and the infrared light absorbent has a coloring opacity of 20 or less, and of phthalocyanine compound and / or naphthalocyanine compound.
Owner:FUJIFILM BUSINESS INNOVATION CORP
Solar control laminates
InactiveUS20080057185A1Reduce transmissionSynthetic resin layered productsOptical articlesInfraredControl layer
Provided is a solar control composition comprising an infrared absorbing phthalocyanine compound or naphthalocyanine compound and a resin having a modulus from 20,000 psi (138 MPa) to 1000 psi (7 MPa) and solar control laminates comprising the solar control composition of the invention.
Owner:WALL JASON S +4
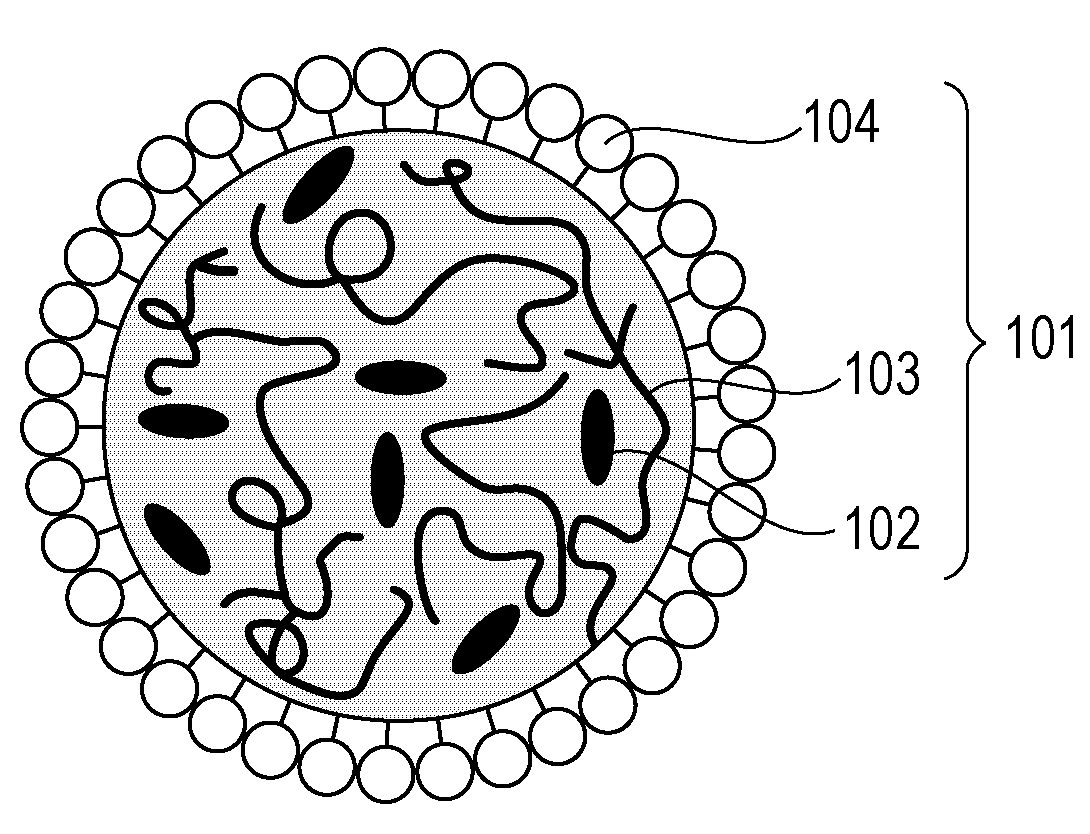
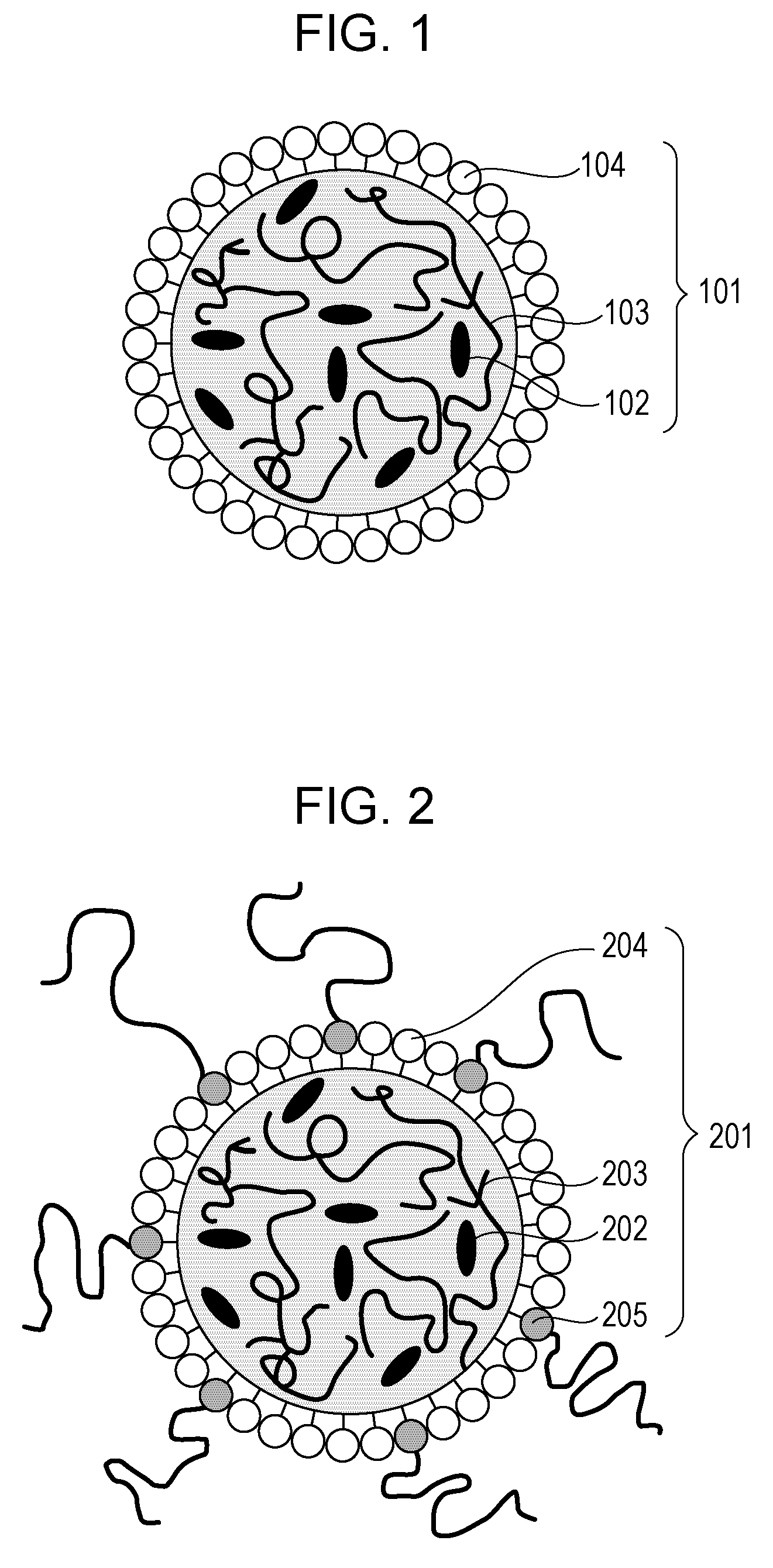
Particles and contrast agent including the same for optical imaging
ActiveUS20130243694A1Reduce leakageHigh tumor accumulationPowder deliveryEchographic/ultrasound-imaging preparationsGlycolic acidNaphthalocyanine
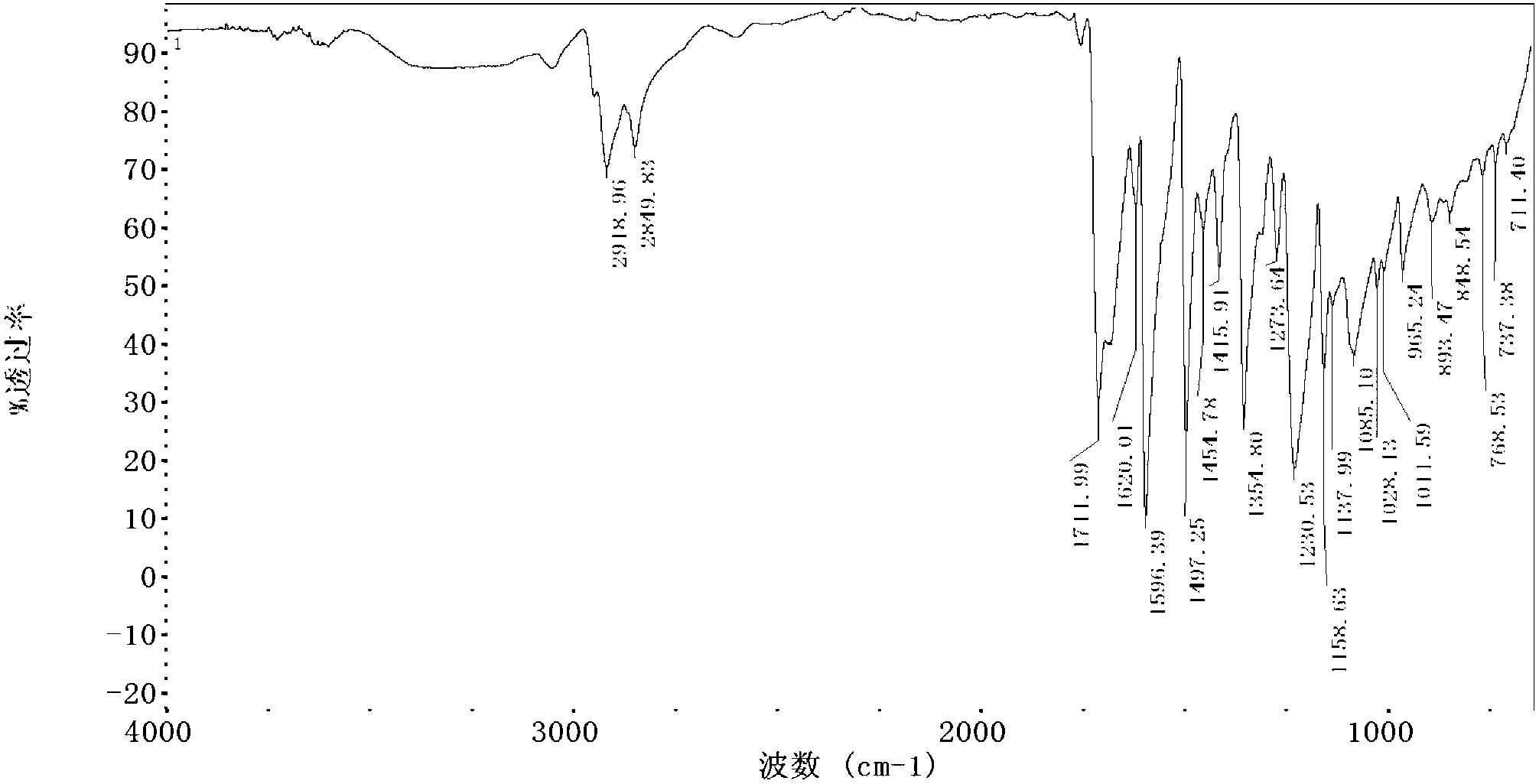
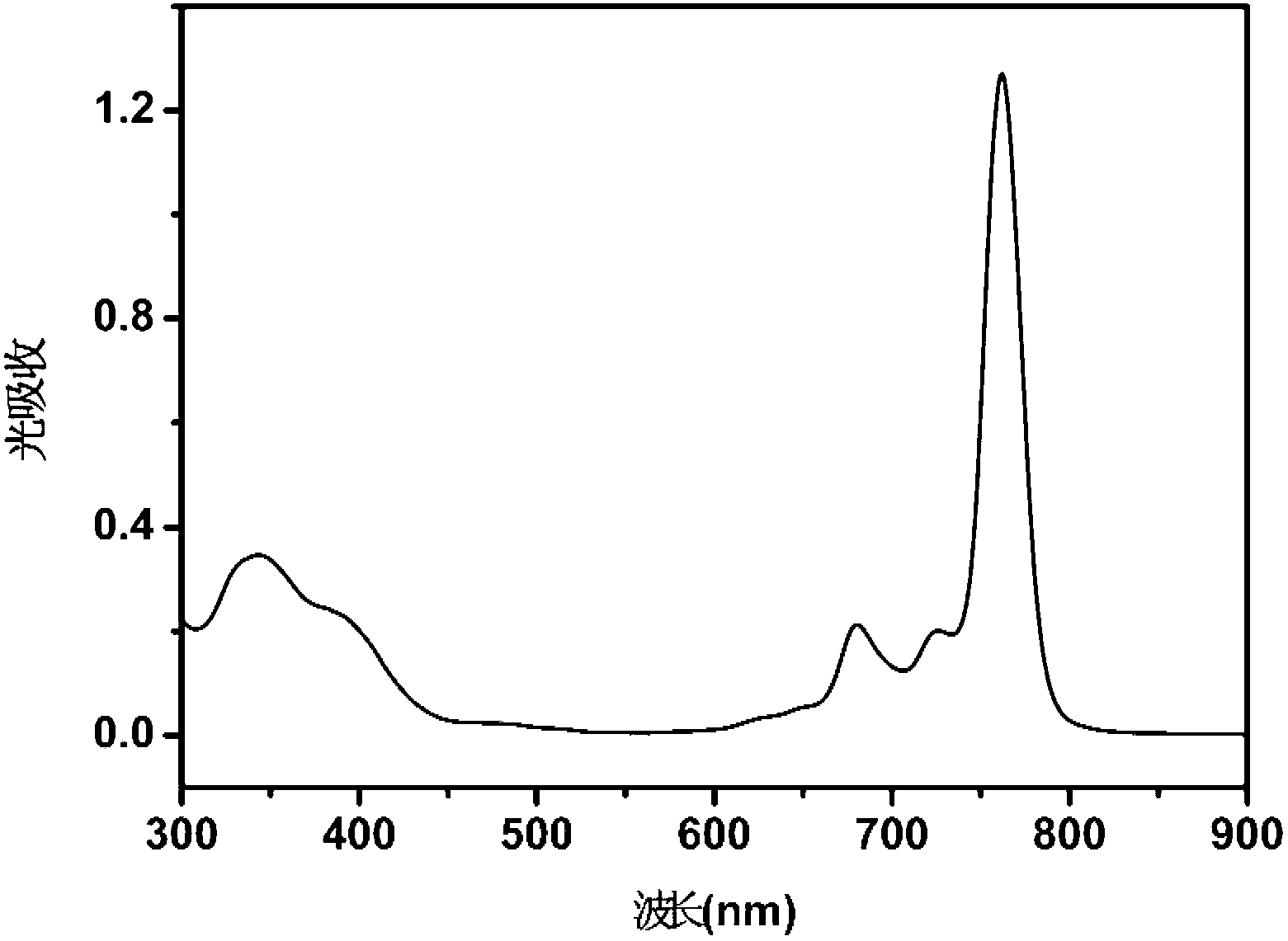
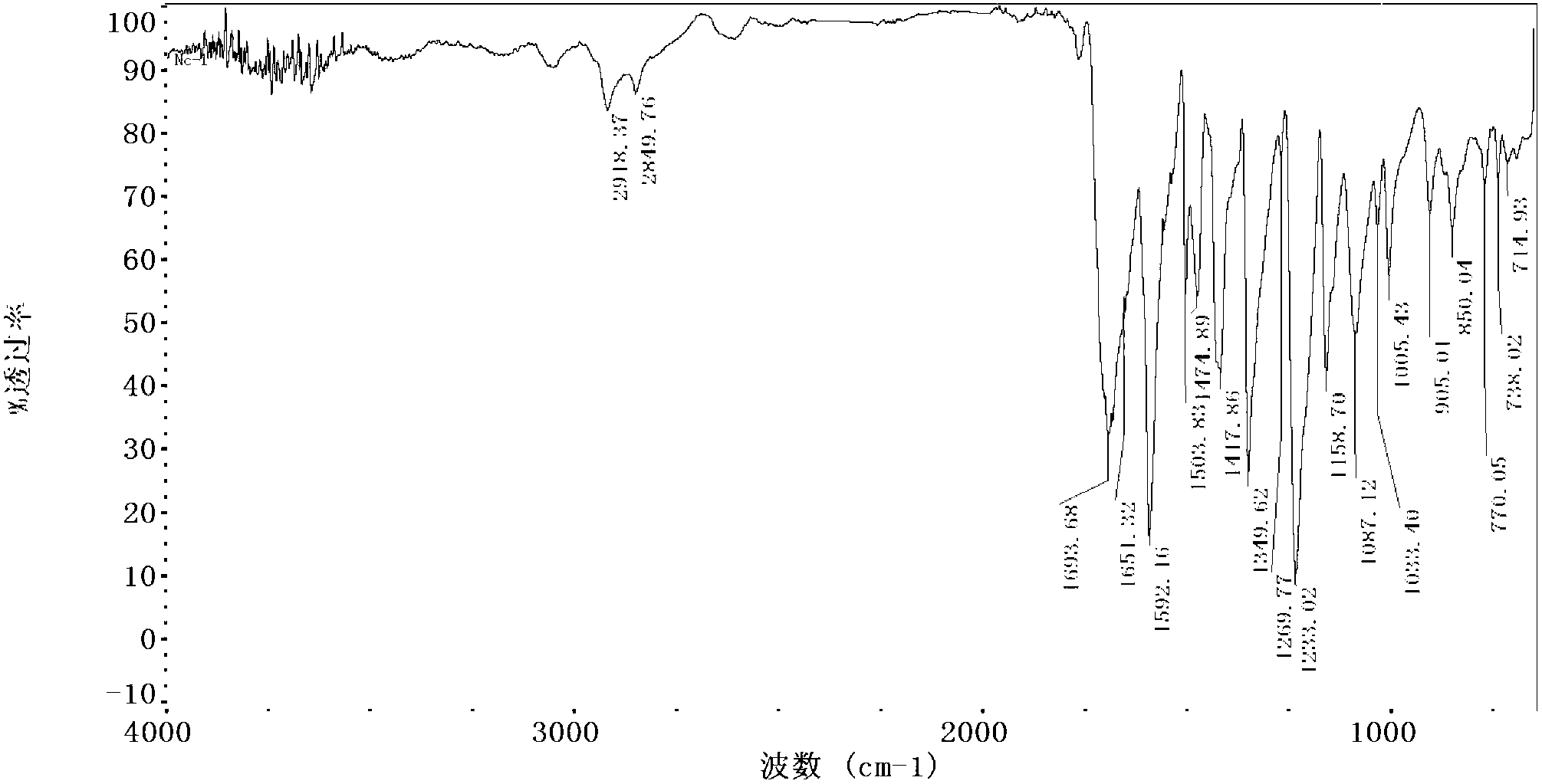
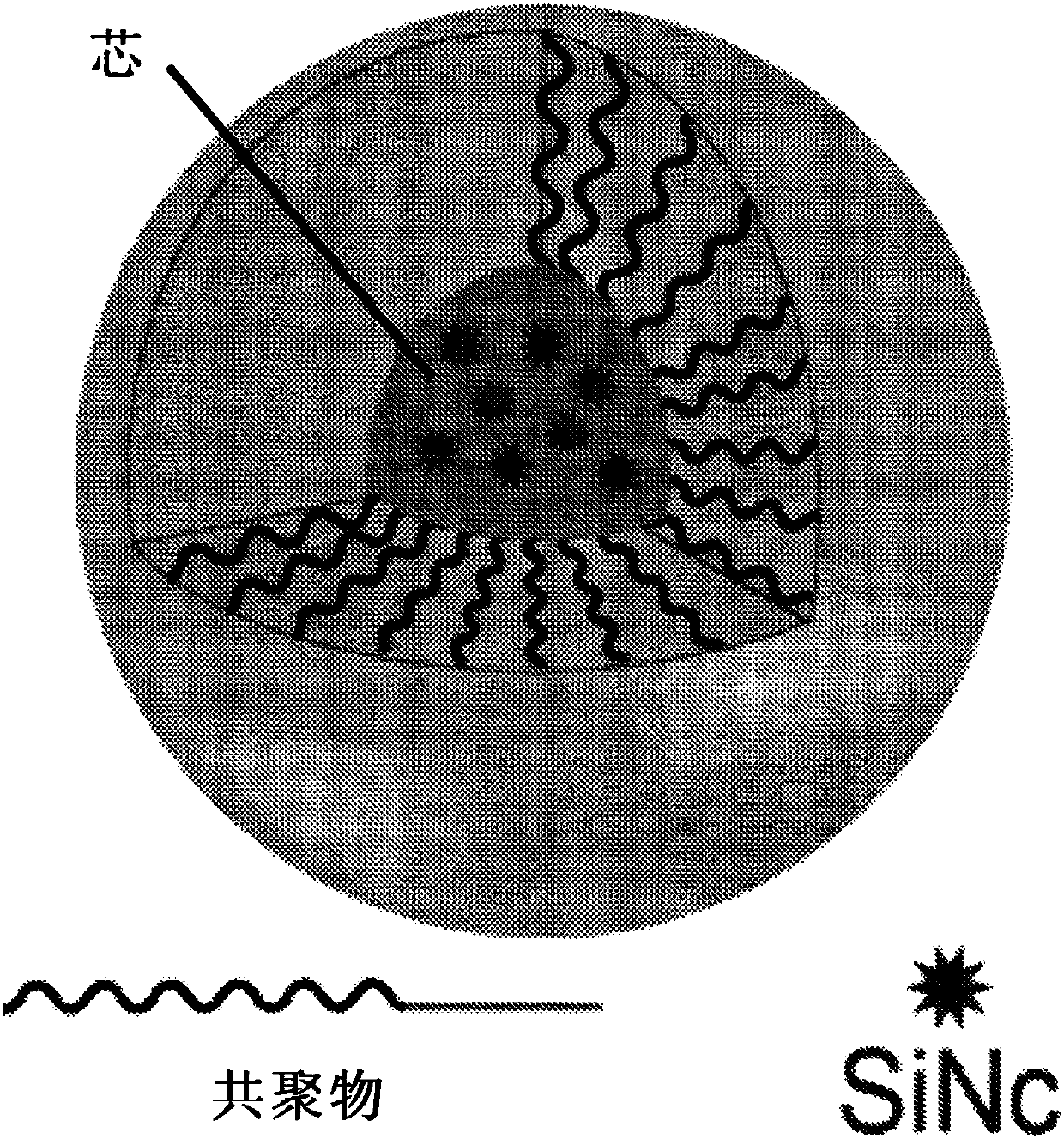
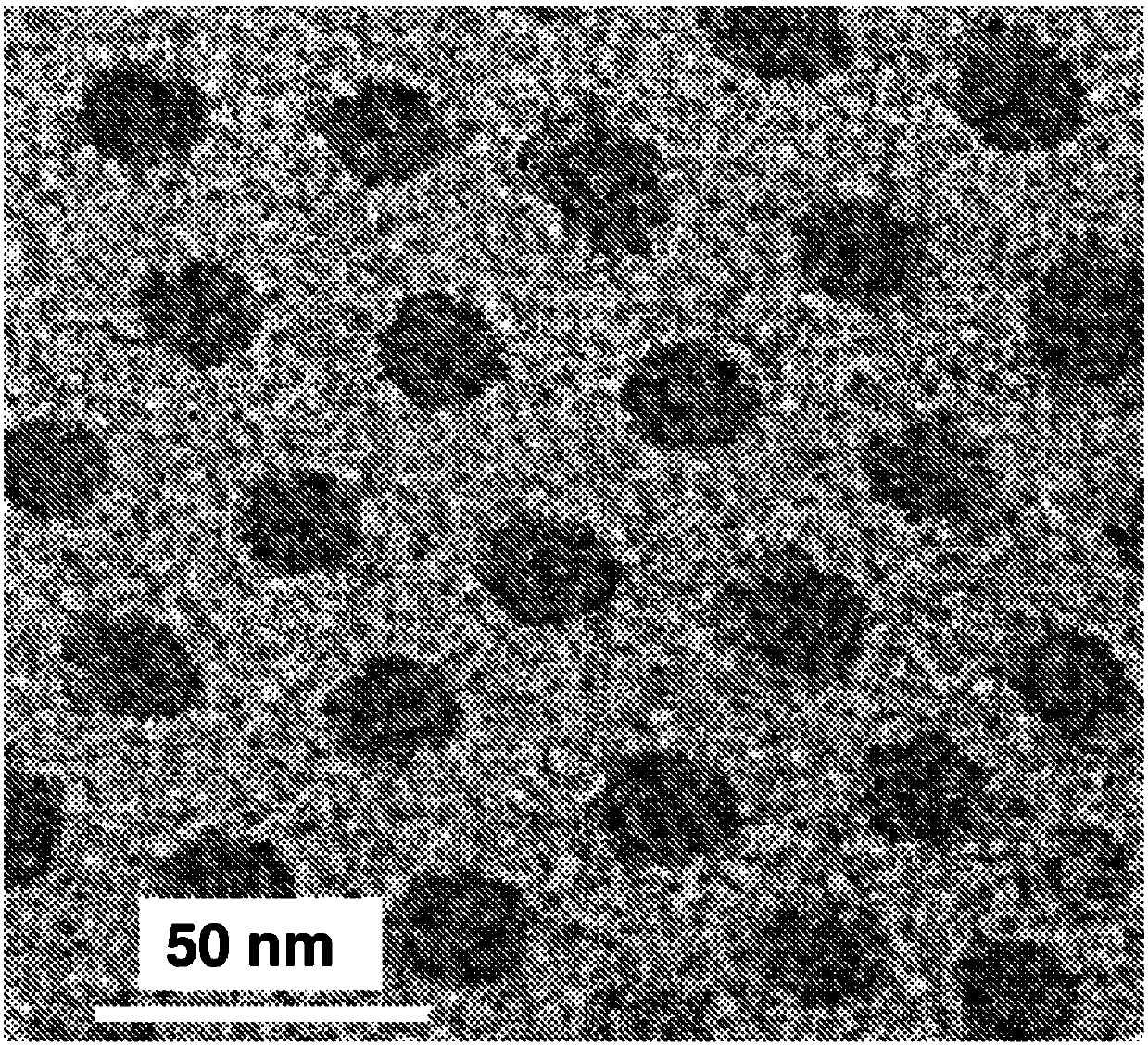
A particle includes a copolymer of lactic acid and glycolic acid, and at least one compound selected from silicon naphthalocyanine and derivatives of silicon naphthalocyanine, in which the particle has a particle size of 10 nm or more and less than 1000 nm.
Owner:CANON KK
Synthetically expedient water-dispersible IR dyes
The present invention provides an IR-absorbing naphthalocyanine dye of formula (I): wherein M is selected from Si(A1)(A2), Ge(A1)(A2), Ga(A1), Mg, Al(A1), TiO, Ti(A1)(A2), ZrO, Zr(A1)(A2), VO, V(A1)(A2), Mn, Mn(A1), Fe, Fe(A1), Co, Ni, Cu, Zn, Sn, Sn(A1)(A2), Pb, Pb(A1)(A2), Pd and Pt; A1 and A2 are axial ligands, which may be the same or different, and are selected from —OH, halogen, —OR3, a hydrophilic ligand and / or a ligand suitable for reducing cofacial interactions; R1 and R2 are selected from H or C1-12alkoxy; R3 is selected from C1-12alkyl, C5-12aryl, C5-12arylalkyl or Si(Rx)(Ry)(Rz); and Rx, Ry and Rz may be the same or different and are selected from C1-12alkyl, C5-12aryl, C5-12arylalkyl, C1-12alkoxy, C5-12aryloxy or C5-12arylalkoxy; W is a hydrophilic group; n1 is 0, 1, 2 or 3; n2 is 0, 1, 2 or 3; n3 is 0, 1, 2 or 3; n4 is 0, 1, 2 or 3; provided that at least one of n1, n2, n3 or n4 is greater than 0. Dyes of this type are especially suitable for use in netpage and Hyperlabel systems.
Owner:BASF AG
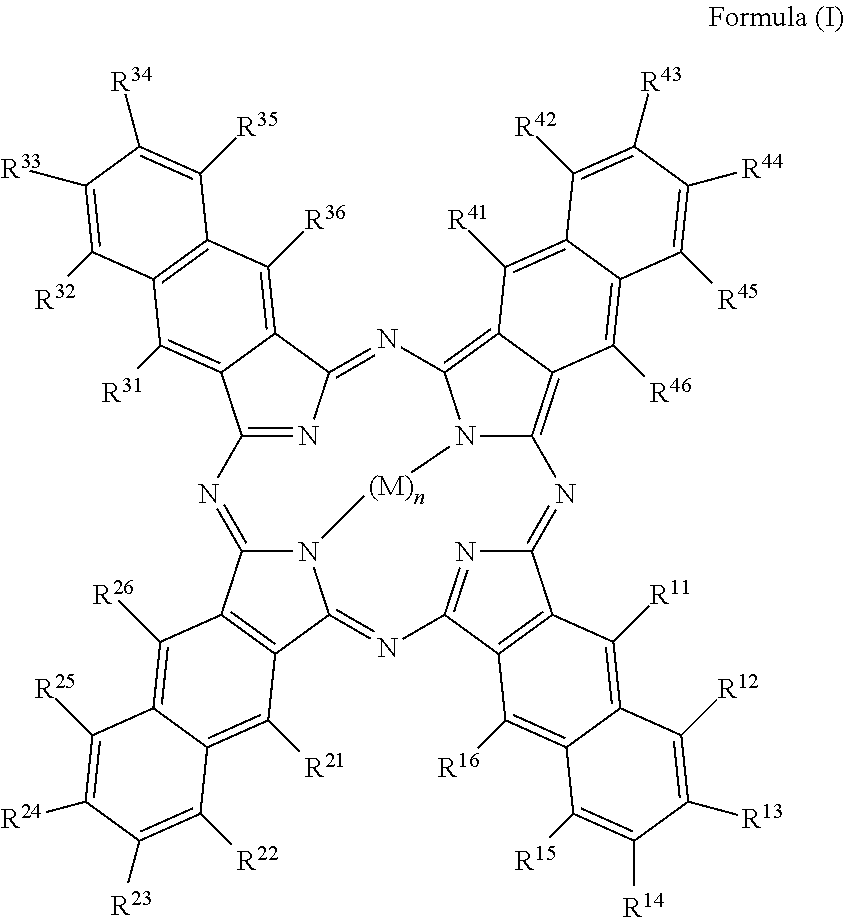
Water-soluble naphthalocyanine base compound, preparation method and application of compound as photosensitizer
InactiveCN103073553AStrong absorption capacityFix compatibility issuesOrganic chemistryEnergy modified materialsPhotodynamic therapyPhotosensitizer
The invention relates to a water-soluble naphthalocyanine base compound, a preparation method and an application of the compound as a photosensitizer. A structural formula of the compound is as Formula (I) as shown in the specification. The compound is a carboxylic acid base modified water-soluble naphthalocyanine metal complex, has a good singlet oxygen occurrence rate, low cell photophobic toxicity, and excellent phototoxicity under irradiation, and can serve as a near-infrared PDT (Photodynamic Therapy) photosensitizer. The invention also provides a preparation method of the compound. The preparation method is simple in technology and wide in application scope.
Owner:SHANDONG UNIV
Composition comprising photosensitive compound in polymeric nanoparticle, and method of using composition
ActiveCN107613975AOrganic active ingredientsPowder deliveryPhthalocyanine derivativesFluorescent imaging
Embodiments of a composition comprising a photosensitive compound and a polymer nanoparticle are disclosed herein. The composition may further comprise a targeting moiety. In some embodiments, the photosensitive compound is a phthalocyanine or phthalocyanine derivative, such as a naphthalocyanine. Upon irradiation with near infrared light, the composition may be used as a fluorescent imaging agentand / or as a phototherapeutic agent, such as for photodynamic and / or photothermal therapies. In certain embodiments, the composition is used to treat certain cancers.
Owner:THE STATE OF OREGON ACTING BY & THROUGH THE OREGON STATE BOARD OF HIGHER EDUCATION ON BEHALF OF OREGON STATE UNIV
Fluorinated silicon phthalocyanines and naphthalocyanines for electrophoretic display
Owner:E INK CALIFORNIA
Azobenzene phi-phi stacked graphene photoresponse material and preparation method thereof
ActiveCN102593360AFast Light Response CapabilityEasy to adjust electrical propertiesFinal product manufactureSolid-state devicesIndiumElectric properties
The invention discloses an azobenzene phi-phi stacked graphene photoresponse material and a preparation method thereof. The phoresponse material is produced by stacking azobenzene 5, 9, 14, 18, 23, 27, 32, 36- octabutoxy-2,3-copper naphthalocyanine. The preparation process comprises the following steps that: the well-prepared oxidized graphene solution is sprayed onto tin indium oxide glass to be thermally treated under the temperature of 120 DEG C, and then hydrazine solution with mass fraction of 80 percent is used for reducing the tin indium oxide glass coated with the oxidized graphene; and then the tin indium oxide glass coated with the reduced graphene is soaked in mixed solution of azobenzene and chloroform, which are uniformly dispersed to prepare the photoresponose material. The prepared azobenzene phi-phi stacked graphene photoresponse material has advantages of easiness in controlling optical properties and electric properties, fast photoelectric conversion speed, apparent photoelectric conversion, simple production process and the like.
Owner:TIANJIN UNIV
Photobleaching compositions effective on dingy fabric
InactiveCN1251126AImprove hydrophobicityOrganic detergent compounding agentsOrganic chemistryBleachPhthalocyanine
Photosensitizing compounds suitable for use as laundry detergent photobleaches are disclosed. The disclosed compounds are phthalocyanine and naphthalocyanines comprising axial moieties selected for their hydrophobic character as measured by their ClogP. Also disclosed are methods for bleaching fabrics and methods for disinfecting hard surfaces.
Owner:CASE WESTERN RESERVE UNIV
Method of proving authenticity, signal conversion method, polymer welding method, method of producing lithographic printing plate, ink for printing, toner, and heat ray-shielding material, each using naphthalocyanine compound, and method of producing naphthalocyanine compound
InactiveUS20110311911A1Improve infrared absorption efficiencyHigh yieldOrganic chemistryReactive dyesHydrogen atomNaphthalocyanine
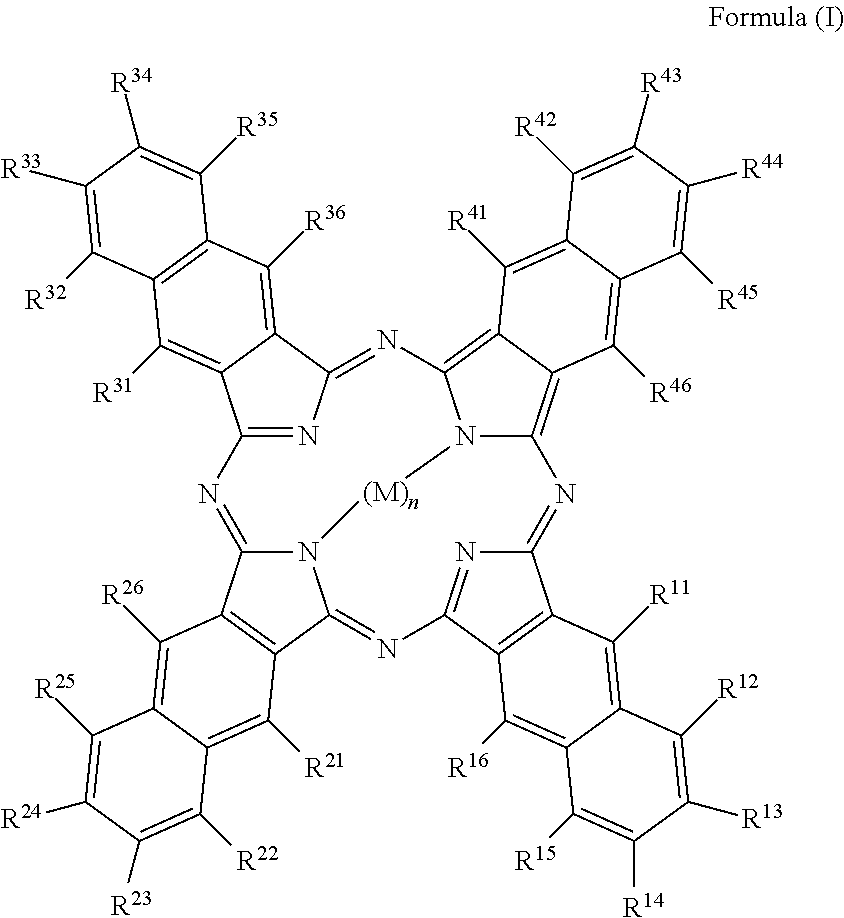
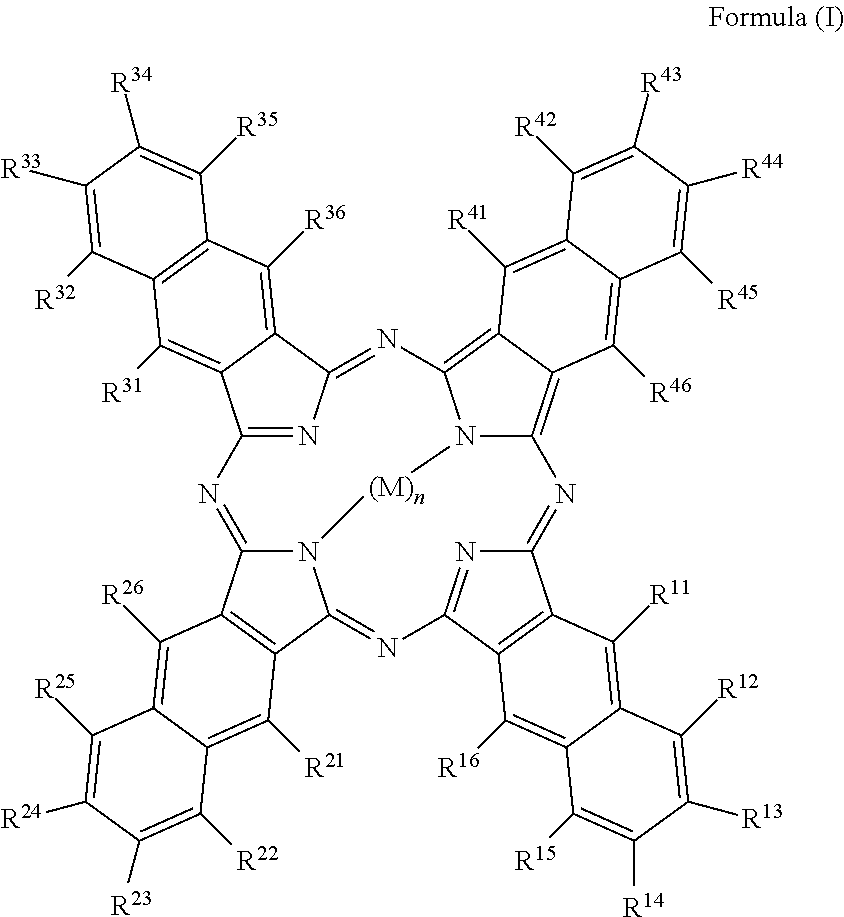
Disclosed is a method of proving the authenticity of goods or a support comprising using a compound represented by Formula (I).wherein, in Formula (I), each of R11 to R46 independently represents a hydrogen atom or a substituent group, wherein when a benzene ring is substituted with any of R11 to R46, any groups adjacent to each other among R11 to R46 may be bonded each other to form a ring; M represents a hydrogen atom, a metal ion, or a group containing a metal ion; and n represents 1 or 2. The infrared absorption efficiency is high and the deterioration in infrared absorption over time is ameliorated. The infrared absorption efficiency is high and the deterioration in infrared absorption over time is ameliorated.
Owner:FUJIFILM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com