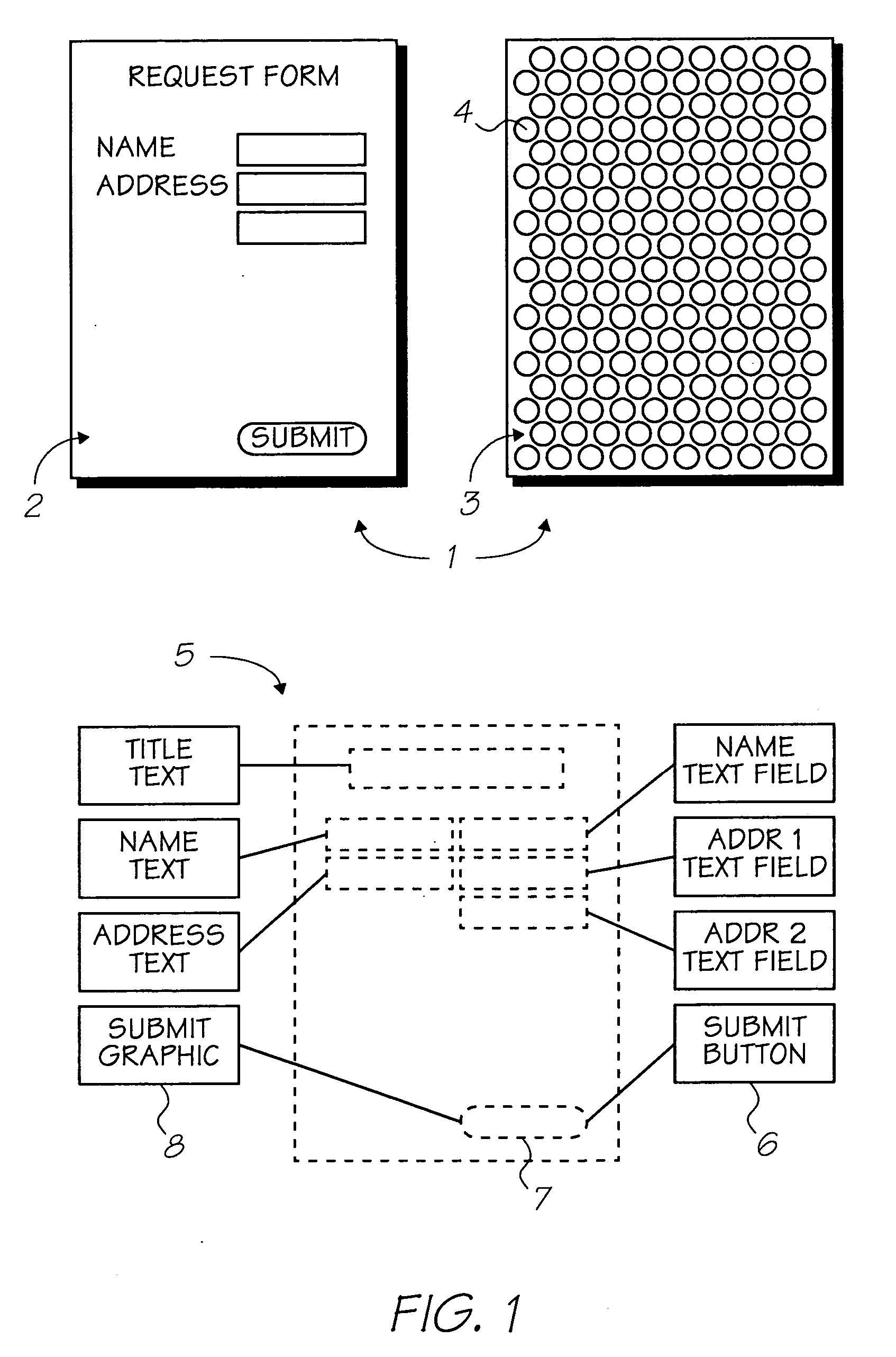
Inkjet ink comprising gallium naphthalocyanine dye
a gallium naphthalocyanine and inkjet technology, applied in the field of infrared (ir) dyes, can solve the problems of no longer preventing scanning of labels, no prior art dyes can be formulated into ink compositions suitable for netpage or hy, and commercially available and/or prior art inks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0395] Hydroxygallium naphthalocyaninetetrasulfonic acid 2 and its corresponding triethylammonium salt 3 were prepared according to the procedure outlined in Scheme 1.
a) 1,2-di(naphthalocyaninatogalliumoxo)ethane (NcGaOCH2CH2OGaNc) 1
[0396] A solution of gallium chloride (0.735 g; 4.18 mmol) in anhydrous toluene (5 mL) was cooled in an ice bath and treated with sodium methoxide portionwise with stirring. Ethylene glycol (5 mL) was then added slowly while cooling the reaction mixture. After stirring for 10 min, naphthalene-1,2-dicarbonitrile (2.98 g; 16.7 mmol) was added all at once as a solid and the resulting slurry was heated initially to distil off the toluene and methanol. Following this, the bath temperature was raised to 190-195 ° C. to induce the cyclotetramerisation to take place. The reaction mixture was maintained at this temperature for 2.5 h and then allowed to cool to room temperature. The resulting green / brown slurry was diluted with absolute ethanol (10 mL) and the ...
example 2
[0399] Hydroxygallium naphthalocyaninetetrasulfonamide 5 was prepared according to the procedure outlined in Scheme 2.
a) hydroxygallium naphthalocyaninetetrasulfonyl chloride (ClSO2)4NcGaOH 4
[0400] Hydroxygallium naphthalocyaninetetrasulfonic acid 2 (374 mg; 0.334 mmol) from Example 1 was suspended in thionyl chloride (3 mL) containing a couple of drops of DMF. The reaction mixture was heated at 60-70° C. (bath) for 2 h under a slow stream of nitrogen, after which time the mixture became homogeneous and dark green in colour. After cooling to room temperature the resulting solution was diluted with chloroform (5 mL) and ether (50 mL) to precipitate the product. The supernatant liquid was decanted and the solid was suspended in ether (50 mL) and filtered off, washing with ether (3×10 mL). After drying under high vacuum the sulfonyl chloride 4 was obtained as a black solid (360 mg; 90%), that was insoluble in water, λmax (DMSO) 803 nm. The absorption spectrum of the sulfonyl chlorid...
example 3
[0410] Hydroxygallium naphthalocyaninetetrasulfonic acid 2, prepared in Example 1, was tested for lightfastness on Reflex Unijet 143 gsm paper, in accordance with the procedure described above. The dye was tested in formulations having dye concentrations of 2.24 mM, 4.49 mM and 7.46 mM. The reflectance spectra measured over time for each dye concentration are shown in FIGS. 33 to 35.
[0411] From these data, the following lightfastness values were estimated:
Hydroxygallium naphthalocyaninetetrasulfonic acid 2:
[0412] 2.24 mM: 39 years
[0413] 4.49 mM: 39 years
[0414] 7.46 mM 92 years
[0415] All lightfastness values were well above accepted industry standards. A significant increase in lightfastness was observed when the concentration of dye is increased to 7.46 mM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission wavelengths | aaaaa | aaaaa |
| emission wavelengths | aaaaa | aaaaa |
| emission wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com