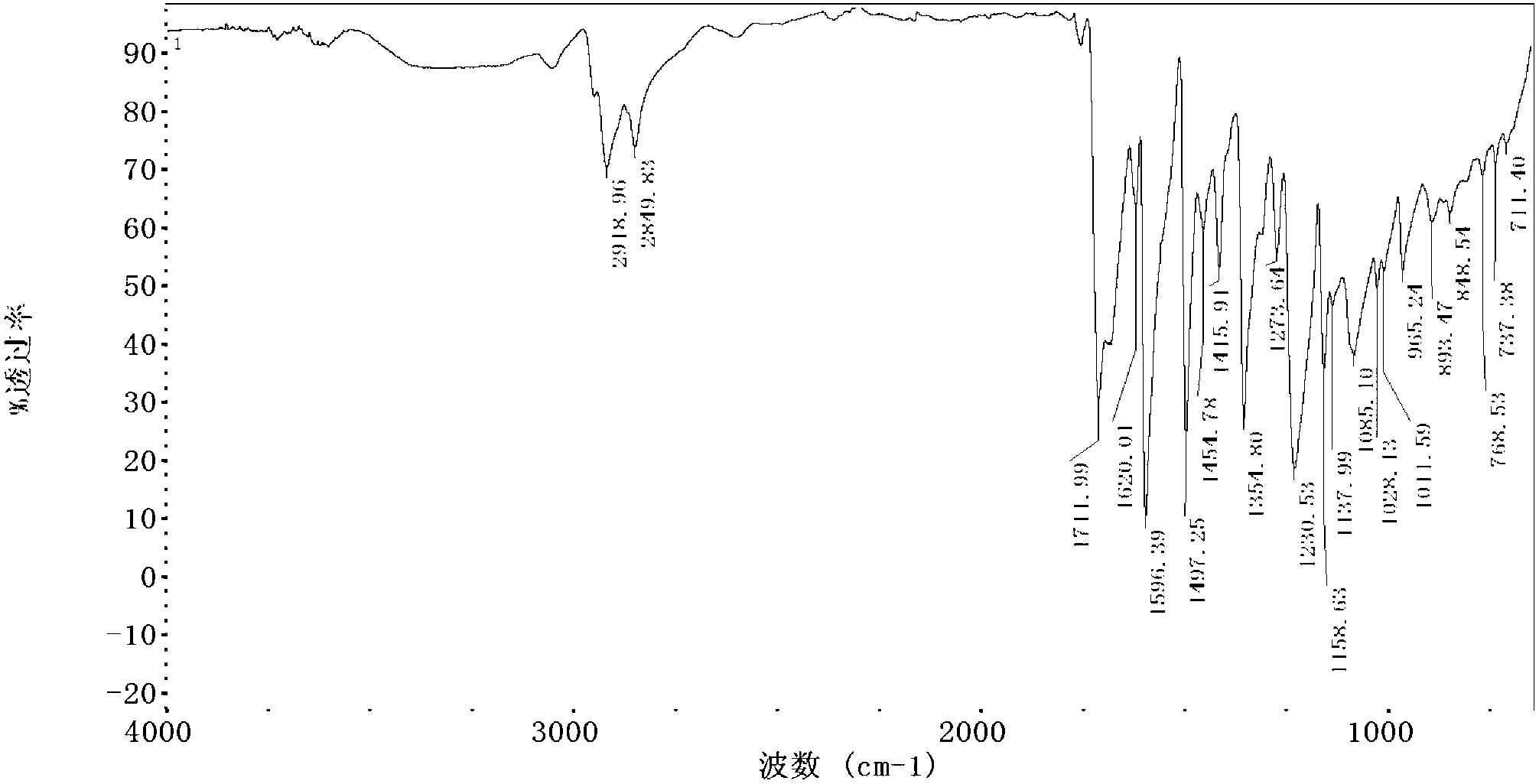
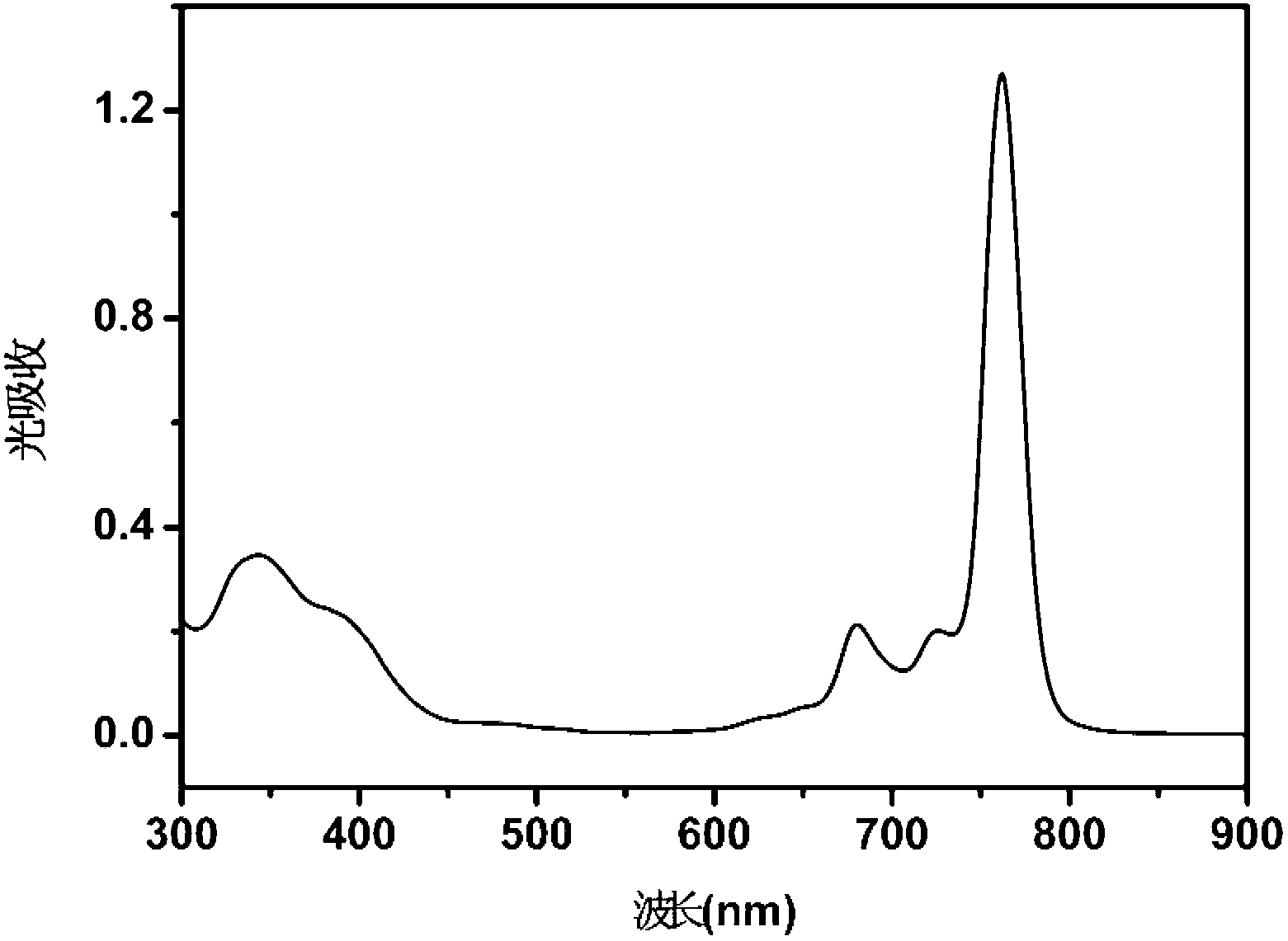
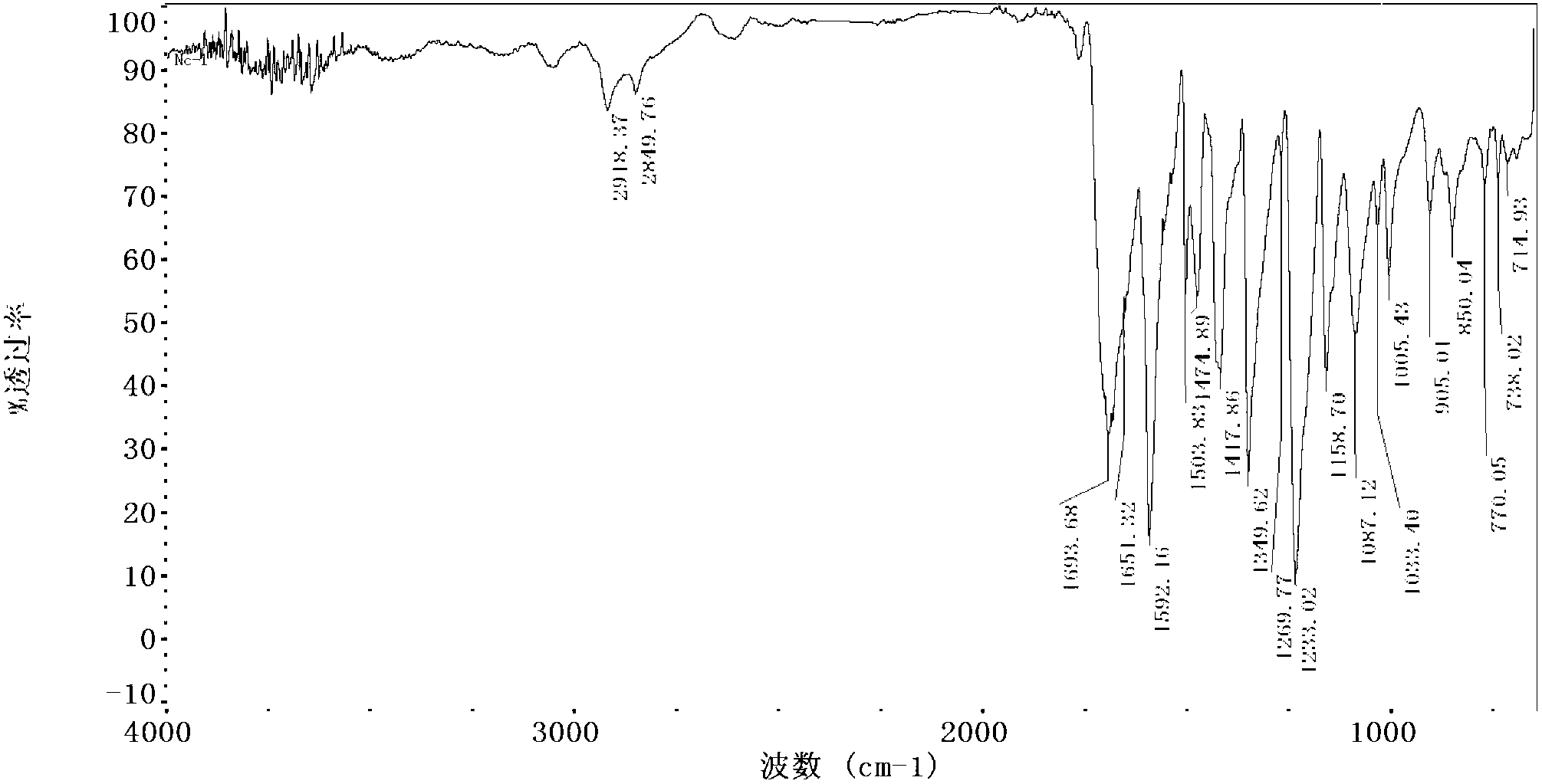
Water-soluble naphthalocyanine base compound, preparation method and application of compound as photosensitizer
A water-soluble, naphthocyanine-based technology, used in organic chemistry, pharmaceutical formulations, drug combinations, etc., can solve the problems of rare naphthocyanine photosensitizers, complex synthesis process, limited application, etc., to achieve good cell killing rate, good affinity Water quality, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Preparation of 6-[(4-methyl carboxylate)phenoxy]-2,3-naphthalene dinitrile (2a)
[0050] Weigh 1g (3.9mmol) of 6-bromo-2,3-naphthalene dinitrile (1a) and 2g (13mmol) of methyl 4-hydroxybenzoate, and 6g of K 2 CO 3 Dissolved in DMF (80mL), stirred at 130°C for 20h. Then the mixture was poured into ice water to obtain a brown precipitate. After filtration, the solid was dissolved in chloroform and washed repeatedly with water three times. The organic layer was collected and the organic solvent was removed under reduced pressure. The crude product was separated by silica gel column chromatography to obtain a yellow solid with a yield of 22%. 1 H NMR (300MHz, CDCl 3 ), δ(ppm): 3.94(S,3H,CH 3 ),7.14(d,2H,Ar-H,J=8.7),7.38(d,1H,Ar-H,J=2.4Hz),7.56(dd,1H,Ar-H,J 1 =2.4Hz,J 2 =9.0Hz),8.0(d,1H,Ar-H,J=9.0Hz),8.12(d,2H,Ar-H,J=8.7Hz),8.18(s,1H,Ar-H),8.33( s,1H,Ar-H). 13 C NMR (300MHz, CDCl 3 )δ (ppm): 52.25, 108.96, 111.14, 114.03, 115.69, 115.84, 119.39, 124.29, 126.87,...
Embodiment 2
[0057] (1) Preparation of 6-bromo-7-[(4-methyl carboxylate)phenoxy]-2,3-naphthalene dinitrile (2b)
[0058] Weigh 1g (3.0mmol) of 6,7-dibromo-2,3-naphthalene dinitrile (2b) and 1.7g (11mmol) of methyl 4-hydroxybenzoate, and 10g of K 2 CO 3 Dissolve in DMF (80mL), and stir the reaction at 85°C for 20h. Then pour the mixture into ice water to obtain a brown precipitate. After filtration, the solid was dissolved in chloroform and washed repeatedly with water three times. The organic layer was collected and the organic solvent was removed under reduced pressure. The crude product was recrystallized in methanol as a white solid with a yield of 75%. 1 H NMR (300MHz, CDCl 3 ,25℃,TMS):δ=3.95(s,3H,CH 3 ),7.13(s,1H,Ar-H),7.16(s,1H,Ar-H),7.30(s,1H,Ar-H),8.13(d,J=2.4Hz,2H,Ar-H) ,8.16(s,1H,Ar-H),8.26(s,1H,Ar-H),8.33ppm(s,1H,Ar-H); 13 C NMR (75MHz, CDCl 3 ,25℃,TMS):δ=52.30,110.14,111.36,114.92,115.41,115.46,119.18,120.29,127.28,130.30,132.26,133.29,133.98,134.40,134.64,155.45,156 m / ...
Embodiment 3
[0064] Embodiment 3: the central ion is M g 2+ Synthesis of Naphthalocyanine Complexes
[0065] As described in Example 1, the difference is that the zinc acetate dihydrate in step (2) is replaced by equimolar magnesium acetate tetrahydrate to obtain the corresponding tetrakis[(4-heptyl carboxylate)phenoxy] Magnesium Naphthalocyanine. Replacing tetrakis[(4-heptyl formate) phenoxy]naphthocyanine magnesium in the step (3) of Example 1 with equimolar tetrakis[(4-heptyl formate)phenoxy]naphthocyanine zinc, resulting product Four [(4-formic acid) phenoxy] naphthalocyanine magnesium. The structure of the resulting product is identical to the naphthalocyanine product described in Example 1 except that the Zn at the naphthalocyanine center is replaced by Mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com