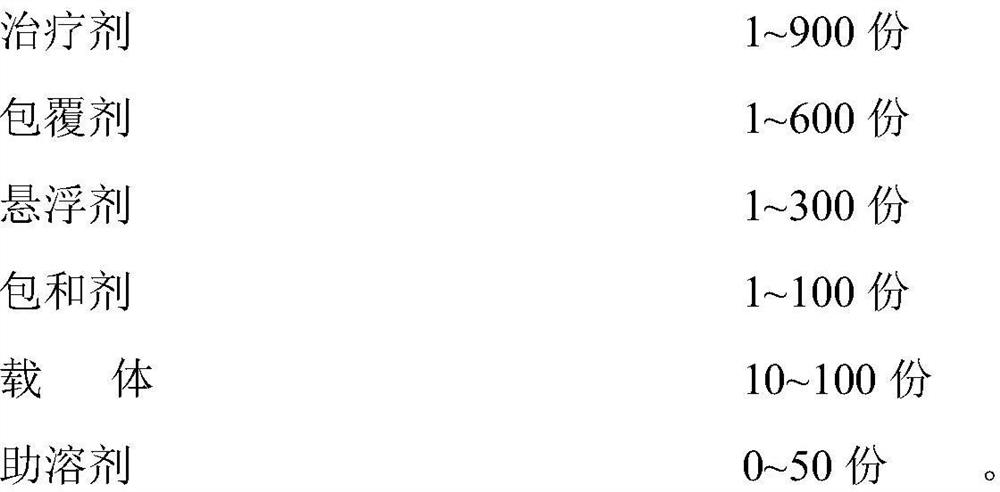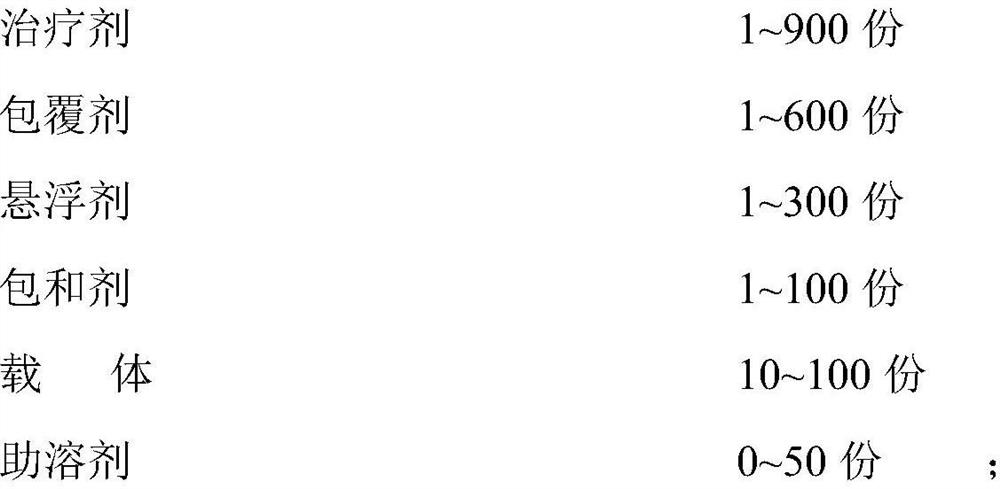Novel preparation of medicinal antiviral drug inhalant and preparation method thereof
A technology for antiviral drugs and inhalants, which is applied in the field of formulations to achieve the effects of reducing the degree of flocculation, promoting dissolution and improving surface properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0063] Embodiment 1-6: Ribavirin dry powder inhaler prescription:
[0064] Table 1-1 Components and dosages of Examples 1-6, specifications: 10-300mg (1 suction)
[0065]
[0066]
[0067] Table 1-2 Components and dosages of comparative examples 1 to 6, specifications: 20-100mg (1 suction)
[0068]
[0069]
[0070] Table 1-3 Performance tests of Examples 1 to 6
[0071]
[0072] Table 1-4 Performance tests of comparative examples 1 to 6
[0073]
[0074]
[0075] Table 1-5 Components and dosages thereof of Examples 7-9 and Comparative Examples 7-8, (1 suction)
[0076]
[0077] Table 1-6 Performance tests of Examples 7-9 and Comparative Examples 7-8
[0078]
Embodiment 10~18
[0080] Table 2-1 Components and dosages of Examples 10-15, specifications: 10-300mg (1 suction)
[0081]
[0082]
[0083] Table 2-2 Performance tests of Examples 10-15
[0084]
[0085] Table 2-3 Components and dosages of Examples 16-18, (1 suction)
[0086]
[0087]
[0088] Table 2-4 Performance tests of Examples 16-18
[0089]
Embodiment 19~21
[0091] Table 3-1 Components and dosages of Examples 19-21, (1 suction)
[0092]
[0093]
[0094] Table 3-2 Performance tests for implementing 19-21
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com