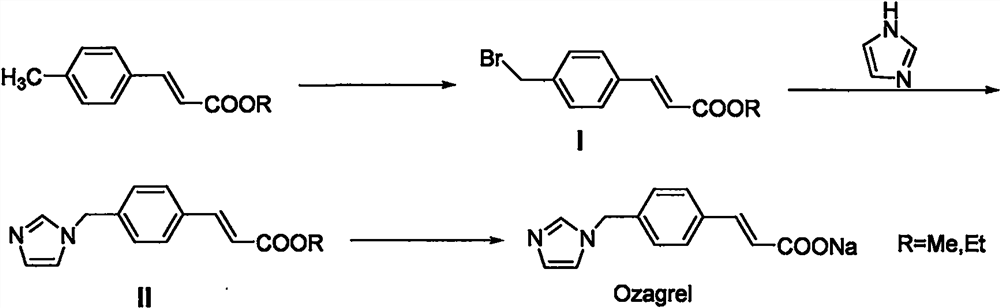
Preparation method of (E)-4-(imidazolyl methyl) cinnamate
A technology of imidazolyl methyl and cinnamate, applied in the direction of organic chemistry and the like, can solve the problems such as the inability to reduce the generation of impurities and the inability to effectively improve the yield, and achieve the advantages of improving convenience, improving quality and improving conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
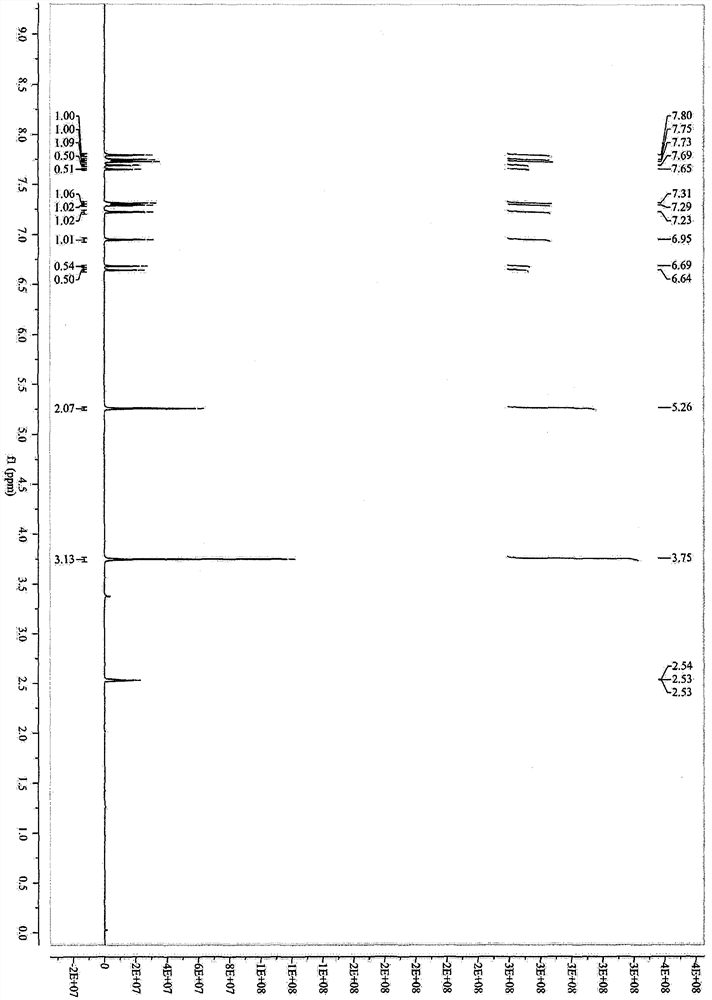
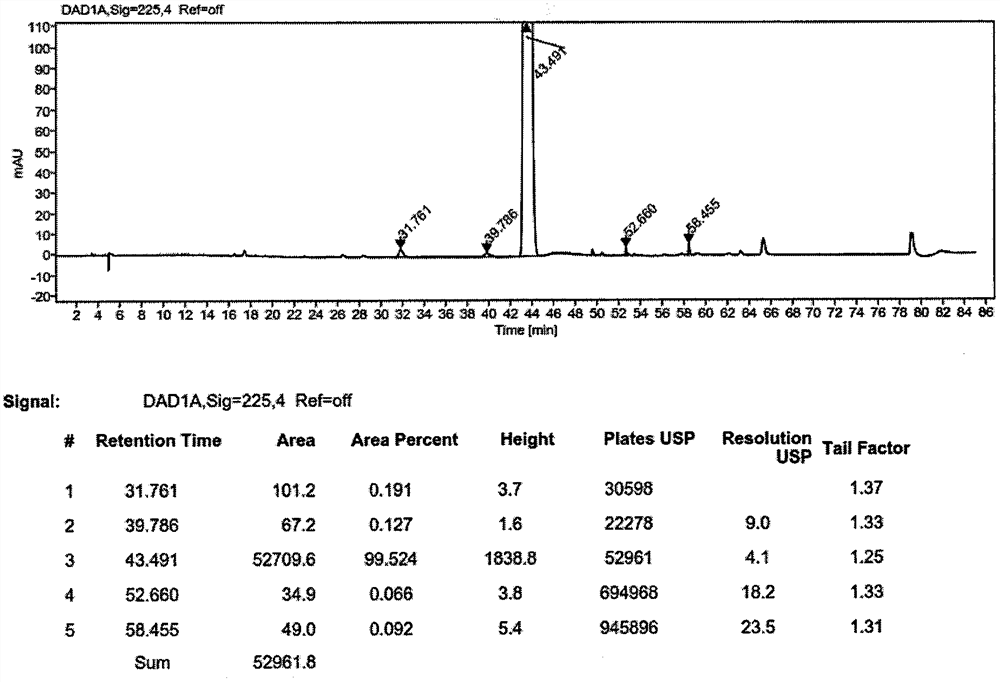
[0037] Add 305g (4.5mol, 1.5eq) of imidazole, 180g (4.5mol, 1.5eq) of sodium hydroxide, and 1.5L of tetrahydrofuran into a 10L reaction flask, and stir at room temperature for 30min to fully react imidazole and sodium hydroxide. Cool down -5°C to 0°C and continue stirring; dissolve 765g (3.0mol, 1.0eq) of methyl 4-bromomethyl cinnamate in 3.0L tetrahydrofuran, stir to dissolve into a solution, and gradually add it dropwise to In the reaction system, after the addition is complete, keep at -5°C to 0°C to continue the reaction for 2 hours, then stop the reaction; add 3N hydrochloric acid to adjust the pH to 6-7, concentrate the reaction solution under reduced pressure, recover the solvent, pour the residue into a 20L transfer barrel, and continue Add 10 L of water, stir and crystallize, filter, and dry the filter cake at 60°C to constant weight to obtain a white solid weighing 690 g, with a yield of 95.0%. 99.52% purity.
Embodiment 2
[0039] Add 205g (3.0mol, 1.2eq) of imidazole, 185g (3.3mol, 1.3eq) of potassium hydroxide, and 1.5L of N,N-dimethylformamide into a 5L reaction flask, and stir at room temperature for 30min to make imidazole and potassium hydroxide fully responsive. Cool down to 0°C to 5°C and continue stirring; dissolve 635g (2.5mol, 1.0eq) of methyl 4-bromomethyl cinnamate in 2.0L N,N-dimethylformamide, stir to dissolve into a solution, and drop Drop the liquid funnel into the reaction system step by step. After the addition, keep the 0℃~5℃ to continue the reaction for 2h, stop the reaction; add 3N hydrochloric acid to adjust the pH to 6~7, pour the reaction solution into a 20L transfer bucket, continue to add 10L of water, and stir Crystallize, filter, and dry the filter cake at 60° C. to constant weight to obtain a white solid weighing 550 g with a yield of 91.2%. 98.88% purity.
Embodiment 3
[0041] Add 340g of imidazole (5.0mol, 2.0eq), 280g of potassium hydroxide (5.0mol, 2.0eq), 1.8L of acetonitrile into a 10L reaction flask, stir and react at room temperature for 1h, the system turns into an orange-red liquid, and control the temperature at 0°C to 5 Continue to stir the reaction for 30 min at ℃; dissolve 670 g (2.5 mol, 1.0 eq) of ethyl 4-bromomethyl cinnamate in 3.0 L of acetonitrile, stir to dissolve, and dissolve ethyl 4-bromomethyl cinnamate in batches The acetonitrile solution was gradually added dropwise into the imidazole / potassium hydroxide reaction system through the dropping funnel. After the addition was complete, the reaction was continued at 0°C to 5°C for 3 hours, and the reaction was monitored by TLC (developing solvent: ethyl acetate / isopropanol / water / ammonia water =7ml / 14ml / 5ml / 0.6ml, UV254nm) to stop the reaction until the raw materials disappear; add acetic acid to adjust the pH to 6-7, concentrate the reaction solution under reduced pressure,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com