Methods and compositions for treating mast cell gastritis, mast cell esophagitis, mast cell enteritis, mast cell duodenitis, and/or mast cell gastroenteritis
A technique for duodenitis and mast cells, which is applied in the fields of treating mast cell gastritis, mast cell esophagitis, mast cell enteritis, mast cell duodenitis and/or mast cell gastroenteritis and compositions, can solve the problem of FDA-approved treatments and more
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0387] Example 1: Phase 1b, open-label, dose-escalation proof-of-concept for evaluating the safety, tolerability and clinical benefit of anti-Siglec-8 antibody therapy in patients with mast cell gastritis and / or gastroenteritis research structure
[0388] Ongoing studies evaluating the efficacy and safety of anti-Siglec-8 antibodies for the treatment of patients with eosinophilic gastritis and / or gastroenteritis have identified the following subpopulation of patients, which although consistent with abdominal pain, nausea and Symptom criteria for / or diarrhea, but without the pre-requisite number of eosinophils in the gastric and / or duodenal mucosa. Instead, these patients were found to have a large number of mast cells (in most cases greater than 30 mast cells / high power field (HPF)) in the gastric and / or duodenal mucosa. Normal levels have been measured to be approximately less than 20 mast cells / HPF (Doyle et al., Am.J.Surg.Pathol. (2014) 38:832-843; Jakate et al., Arch.Path...
Embodiment 2
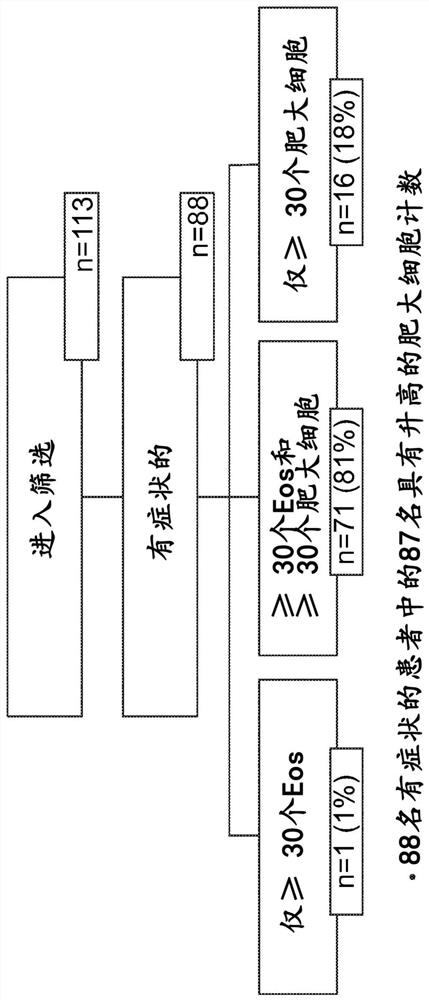
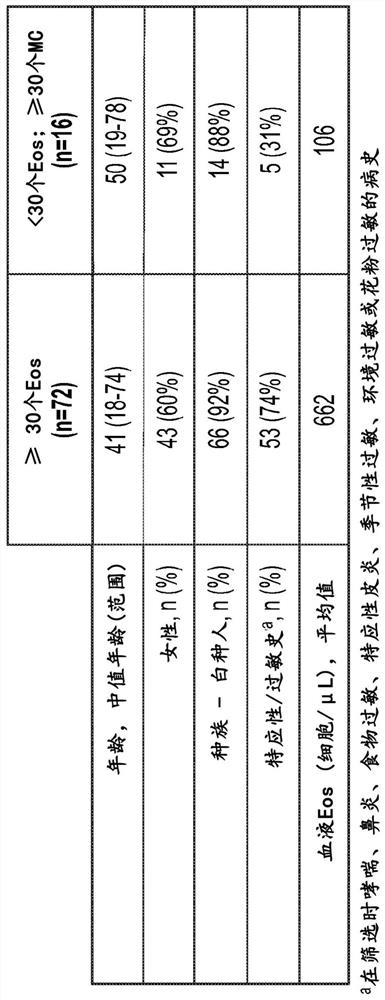
[0442] Example 2: Symptomatic patients suspected of having eosinophilic gastritis and / or enteritis have elevated mucosal mast cell counts in the absence of hypereosinophilia
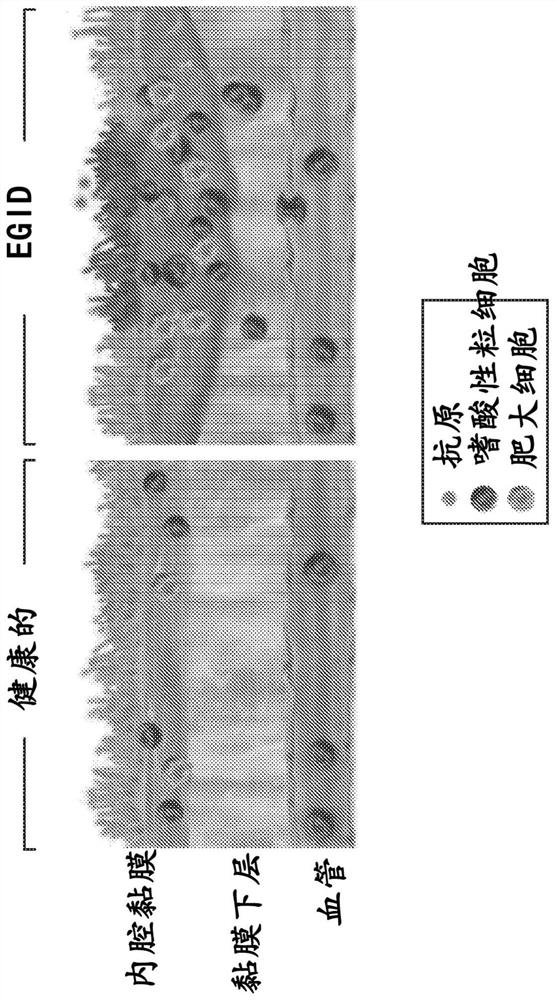
[0443] Pathological accumulation and hyperactivation of eosinophils are implicated in a variety of chronic inflammatory diseases of the GI tract (Figure 1), including eosinophilic esophagitis (EoE), gastritis (EG), , enteritis (EEn) and colitis (collectively known as eosinophilic gastrointestinal disease, EGID). Quality of life in patients with EGID is reduced due to debilitating symptoms such as choking / dysphagia, abdominal pain, nausea, vomiting, and diarrhea.
[0444] Although the pathogenesis of EGID is historically thought to be driven by eosinophils, mast cells have also been shown to be elevated in EoE (Caldwell et al. (2014) J. Allergy Clin. Immunol. 134:1114-1124; Youngblood et al. (2019) JCI Insight 4(19)). However, the role of mast cells in EGID (especially other than EoE) has not been estab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com