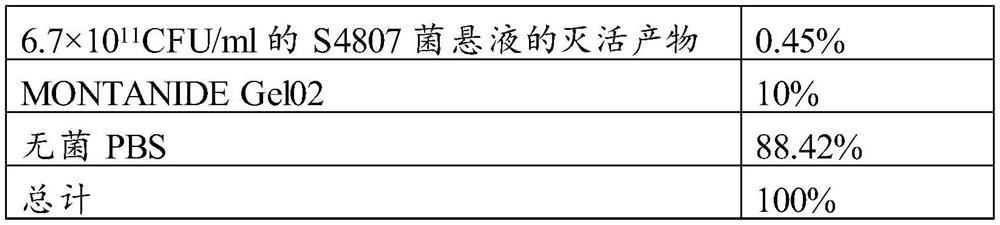
Streptococcus strain and vaccine for preventing and treating swine streptococcosis
A technology of Streptococcus suis and strain, applied in the field of veterinary medicine, can solve the problems of non-preventive effect and great stress on pigs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the isolation of bacterial strain
[0055] 1. Disease material
[0056] Collect brains, lungs, trachea, heart blood, joint fluid, etc. from diseased pigs and dead pigs with dyspnea, sepsis, arthritis and neurological symptoms in pig farms.
[0057] 2. Isolation and culture of bacteria
[0058] Aseptically operate, evenly spread the suspected suis streptococcus disease material on the TSA solid medium containing 10% fetal bovine serum. After culturing for 24 hours in a 37°C incubator. Pick a suspicious single streptococcal colony for purification.
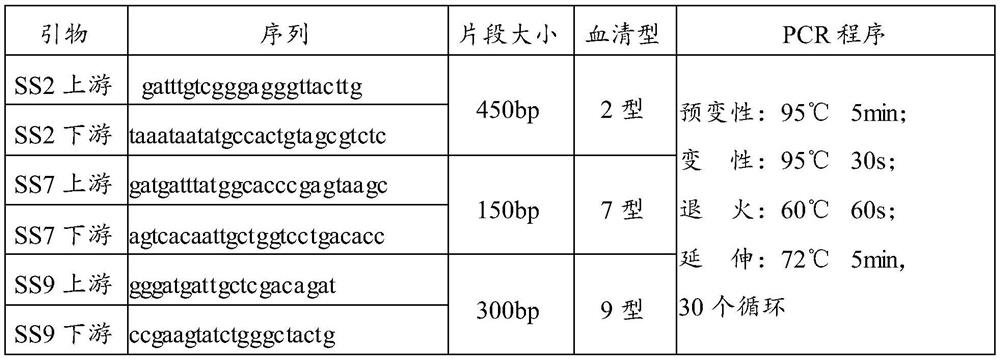
[0059] 3. Serotyping
[0060] The serotypes were identified according to the primer sequences and PCR program in the following table.
[0061] Table 1
[0062]
[0063] 4. Strain preservation
[0064] The correctly identified Streptococcus suis type 2 was named as S4807 strain; Streptococcus suis type 7 was named S8980 strain; The correctly identified Streptococcus suis was added to skimmed milk powder ...
Embodiment 2
[0065] Embodiment 2: Streptococcus suis type 2, type 7 and type 9 antigen preparation
[0066] 1. Source of the strain
[0067] The selected Streptococcus suis type 2 is S4807 strain, and the preservation number is CGMCC No.22747.
[0068] The selected Streptococcus suis type 7 is S8980 strain, and the preservation number is CGMCC No.22748.
[0069] The selected Streptococcus suis type 9 is S1802 strain, and the preservation number is CGMCC No.22749.
[0070] 2. Preparation and inspection of vaccine semi-finished products
[0071] 2.1 Preparation of seed batches for production
[0072] 2.1.1 Propagation of primary seeds
[0073] Dilute S4807 strains, S8980 strains and S1802 freeze-dried bacteria with TSA liquid medium, respectively streak inoculate them with TSB solid medium (containing 10% fetal bovine serum), culture at 37°C for 24 hours, and select off-white transparent small colonies Inoculate in TSB solid medium (containing 10% fetal bovine serum), and cultivate at 3...
Embodiment 4
[0104] Example 4 Safety Inspection
[0105] 1 test plan
[0106] 1.1 Materials: Vaccine A, Vaccine B, Vaccine C, Vaccine D
[0107] 1.2 Method: 25 piglets aged 3 to 4 weeks were selected and randomly divided into 5 groups with 5 piglets in each group. Groups 1 to 4 were intramuscularly injected with overdose of vaccines A to D in the neck, 4ml / head, and group 5 was the control group without immunization, and they were continuously observed for 14 days.
[0108] 2 test results
[0109] Table 7
[0110]
[0111] The results of overdose injection of piglets aged 3 to 4 weeks are shown in Table 7. The results showed that the feed intake, energy, and body temperature of the vaccines A to D were normal, and there were no adverse reactions at the injection site, and no other visible abnormal clinical manifestations; , each organ had no abnormal lesions. It shows that the vaccine is safe and has no side effects.
[0112] Embodiment 4 effectiveness test
[0113] 1. Experiment...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap