Methods and compositions for genetically modifying lymphocytes in blood or in enriched pbmcs
A gene modification, blood cell technology, applied in the field of immunology, can solve the problem of inactivation of retroviral particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0568] Example 1. Materials and methods for transduction experiments
[0569] This example provides the materials and methods used in the experiments disclosed in the subsequent examples herein.
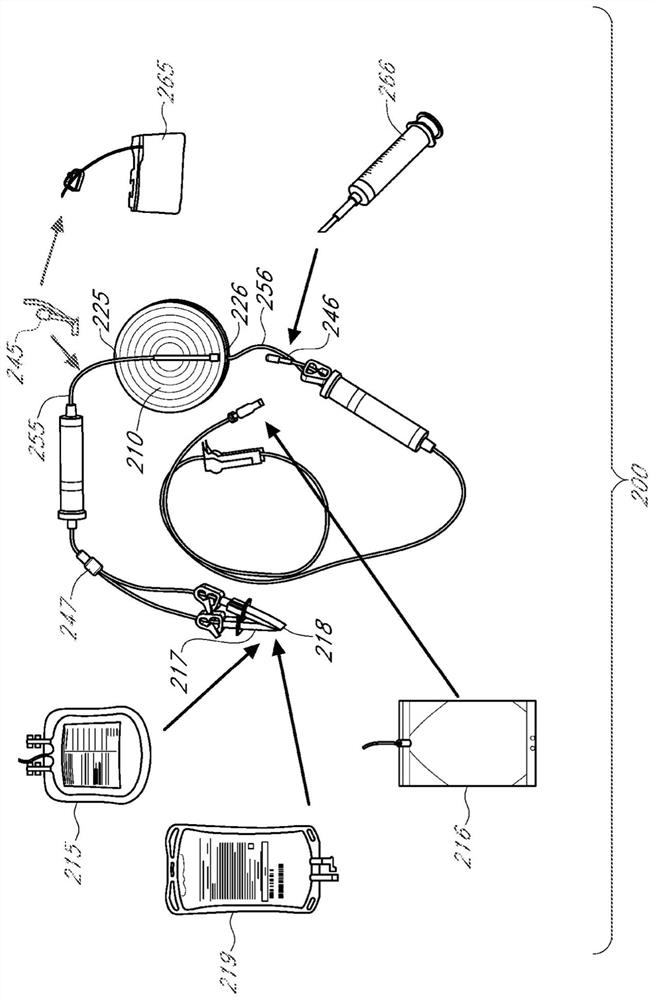
[0570] Recombinant lentiviral particles are produced by transient transfection.
[0571] Unless otherwise stated, 293T cells (Lenti-X TM 293T, Clontech) suitable for passing in Freestyle TM Suspension cultures (referred to as F1XT cells) were performed with continuous growth in 293 expression medium (ThermoFisher Scientific) and used as packaging cells in the experiments herein.
[0572] It should be noted that a typical 4-vector packaging system includes 3 packaging plastids encoding (i) gag / pol, (ii) rev, and (iii) a pseudotyping element such as VSV-G. The fourth vector of this packaging system is a genomic plastid, a third-generation lentiviral expression vector encoding one or more related genes (with a deletion in the 3'LTR that causes self-inactivation). For transfections u...
example 2
[0580] Example 2. Exposure to retroviral particles pseudotyped VSV-G or influenza HA and NA for 4 hours and optionally with an envelope derived from VSV-G, MV or MuLV and furthermore optionally anti-CD3 on its surface Transduction efficiency of scFv co-pseudotyped unstimulated PBMCs.
[0581] In this example, lentiviral particles pseudotyped or co-pseudotyped by various envelope proteins and optionally displaying T cell activation elements were exposed to unstimulated human PBMC for 4 hours and transduction efficiency assessed .
[0582] Production of recombinant lentiviral particles in F1XT cells. Cells were transiently transfected with PEI with genomic plastids and separate packaging and envelope plastids encoding gag / pol, rev. For some samples, the transfection reaction mixture also included plastids encoding UCHT1 scFvFc-GPI, a co-pseudotyped envelope, or a co-pseudotyped envelope fused to anti-CD3 scFv. In this example, the genomic plastid used for the sample was F1-0-...
example 3
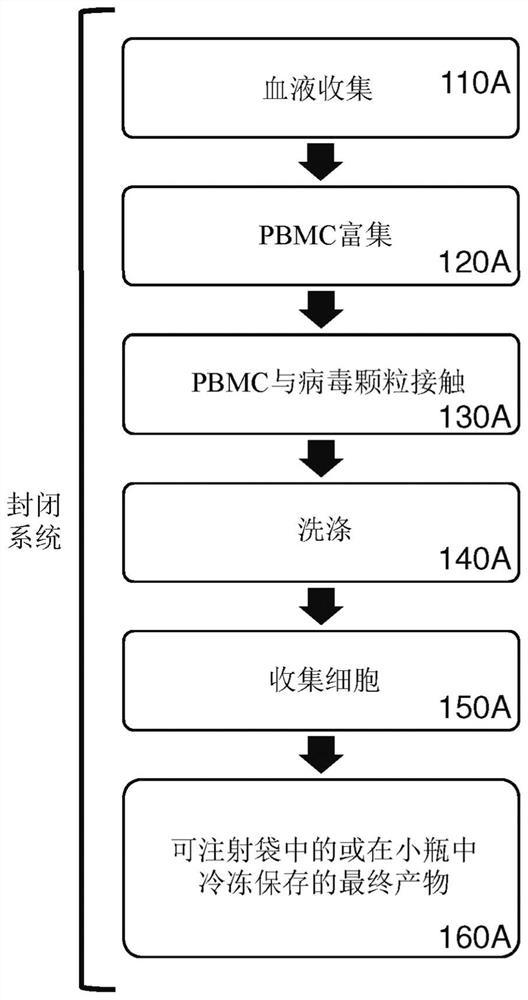
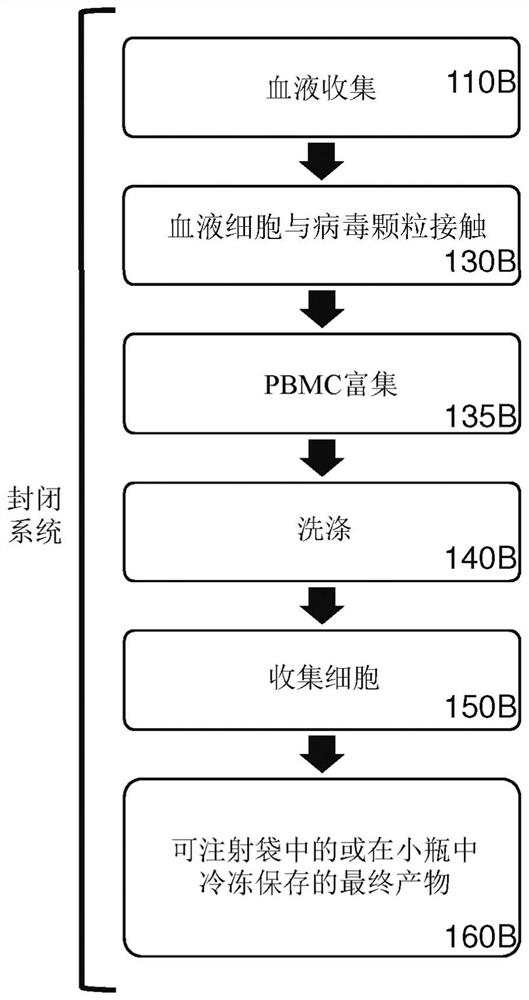
[0587] Example 3. Efficient genetic modification of resting lymphocytes by exposing whole blood to recombinant retroviral particles for 4 hours, followed by a PBMC enrichment procedure.
[0588]In this example, unstimulated human T cells and NKT cells were efficiently genetically modified by a 4-hour incubation of a reaction mixture comprising whole blood and retroviral particles pseudotyped by VSV-G and Displays T cell activation elements on its surface. Next, PBMCs were isolated from the transduction reaction mixture using a conventional density gradient centrifugation-based PBMC enrichment procedure. Transduction of CD3+ cells was assessed by expression of the eTag transgene using flow cytometry.
[0589] Purification using depth filtration The following lentiviral particles were used in this example: F1-3-23 (F1-3-23G) pseudotyped by VSV-G; and F1-3-23G pseudotyped by VSV-G and displaying T cell activation elements F1-3-23 of UCHT1-scFvFc-GPI (F1-3-23GU).
[0590] 10 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com