Methods and compositions for transducing and expanding lymphocytes and regulating the activity thereof
A kind of cell, NK cell technology, applied in the field of immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
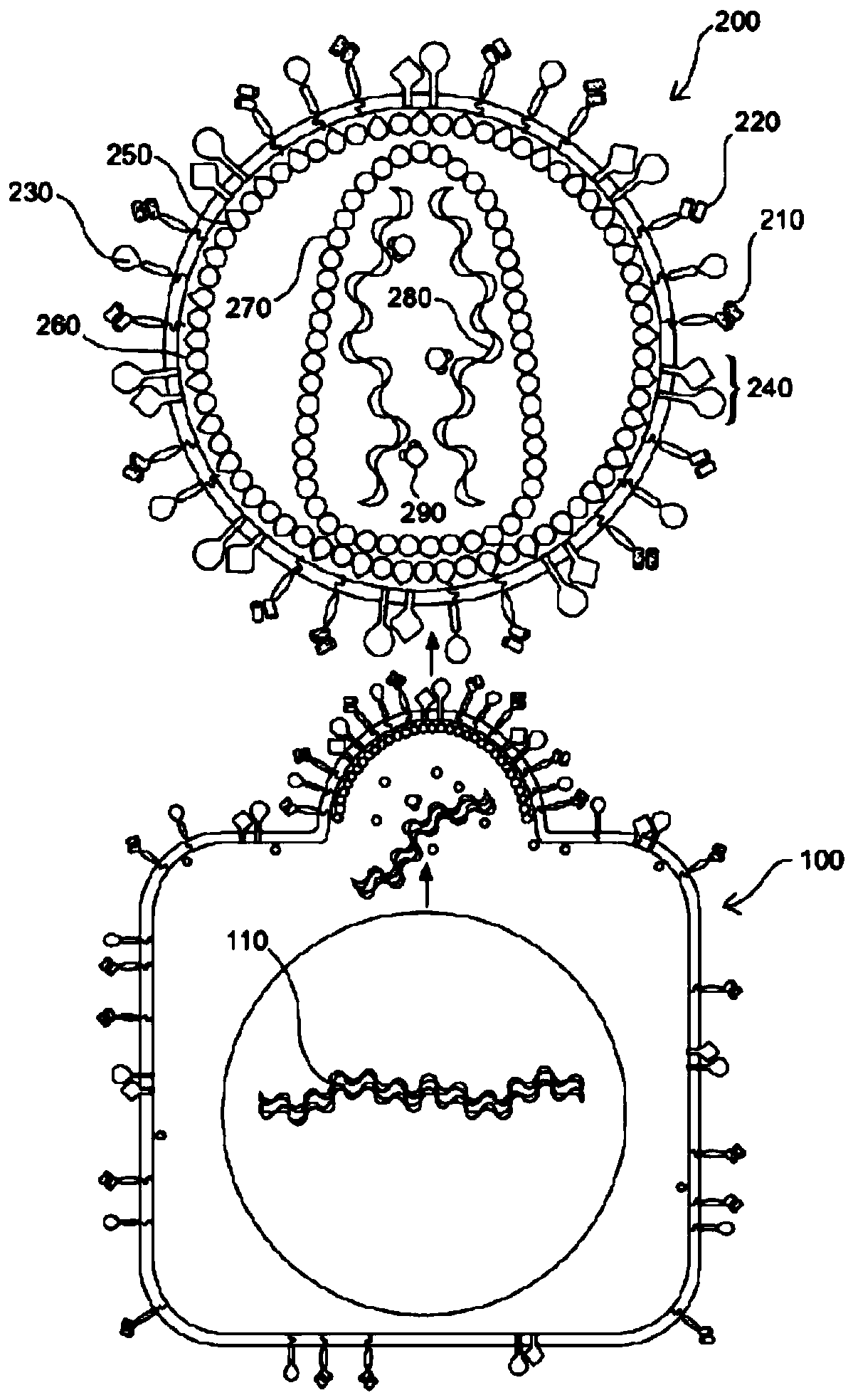
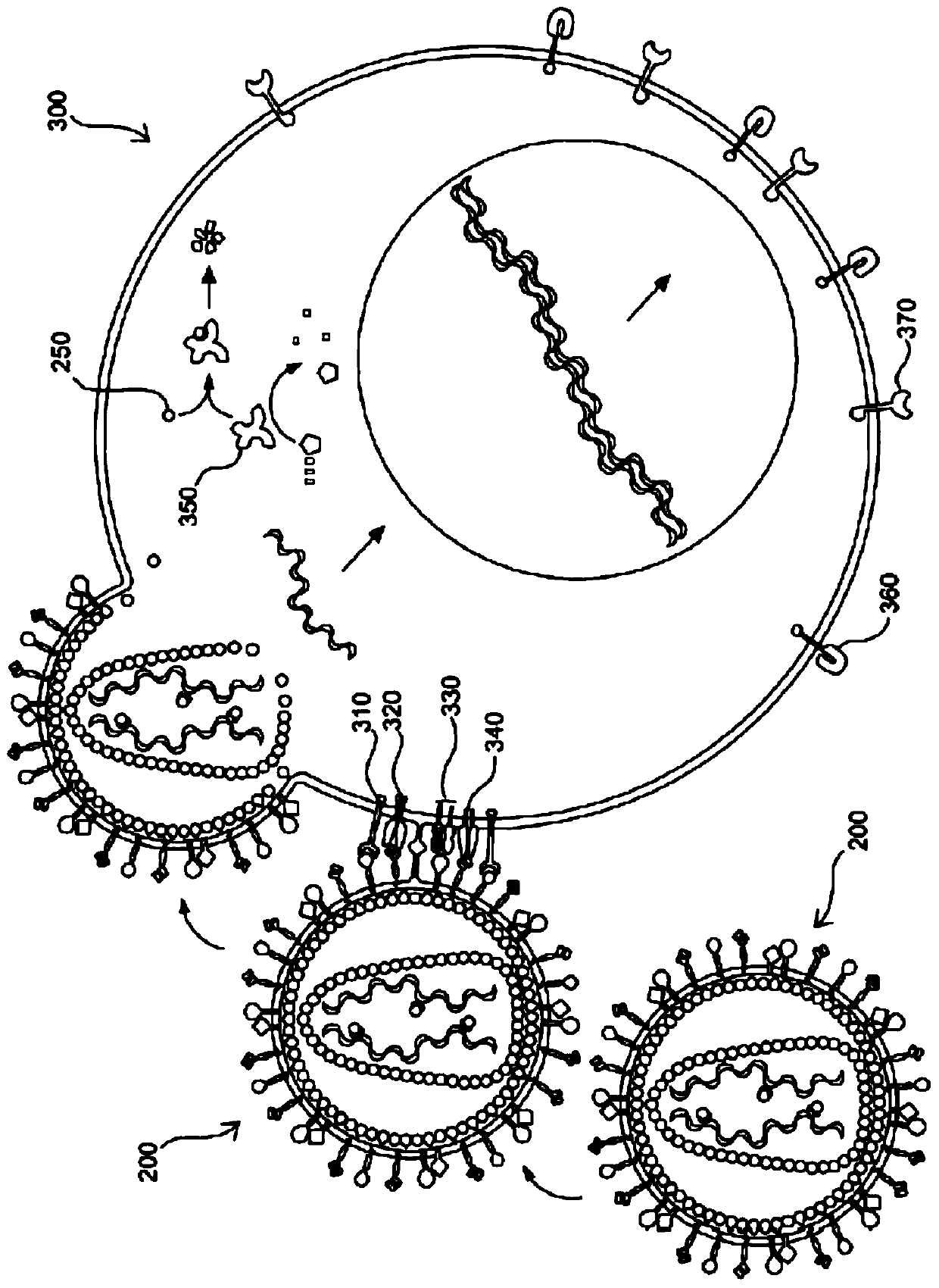
[0099] The present invention overcomes the challenges of the prior art by providing improved methods and compositions for genetically modifying lymphocytes, such as NK cells, and in illustrative embodiments T cells. For example, some of these methods do not include preactivating lymphocytes, and some of these methods are performed in less time than previous methods. Additionally, compositions are provided that have many uses, including their use in these improved methods. Some of these compositions are genetically modified lymphocytes with improved proliferation and survival qualities, including when cultured in vitro, eg, in the absence of growth factors. These genetically modified lymphocytes will have uses such as: as a research tool to better understand the factors that affect T cell proliferation and survival; Production of factors such as growth factors and immunomodulators.
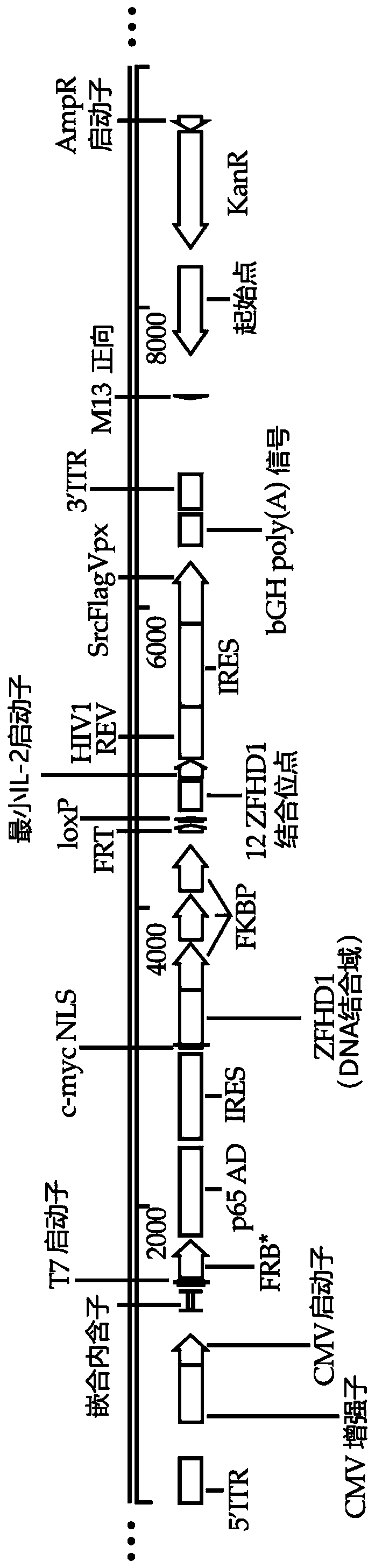
[0100] Some embodiments provided herein are methods for performing adoptive cell therapy comp...
Embodiment 1
[1199] Example 1. Generation of riboswitches specifically responsive to nucleoside analog antiviral drugs.
[1200] This example provides a method for screening libraries based on natural structural riboswitches that bind guanosine and deoxyguanosine. These riboswitches are used as a framework to develop a biased library for selection of aptamers that specifically bind ligand nucleoside analogs. Previously, isothermal titration calorimetry has been used to show that these natural riboswitches bind to their natural ligands. Additional testing revealed that the deoxyguanosine switch also weakly interacts with nucleoside analogs (acyclovir and penciclovir), leading to the redesign of this sequence into a new library. The single-stranded region of the riboswitch is used to specifically respond to selected mutations and variant sequences of acyclovir or penciclovir.
[1201] Material
[1202] Order selected components from Sigma-Aldrich (St. Louis, MO): Guanine, Guanosine, Deoxy...
Embodiment 2
[1233] Example 2. Isolation of conditional scFv's
[1234]The pool of potential splice sites was removed, and tumor antigen-specific scFv's were synthesized using overlapping oligomeric synthesis and cloned into a CAR shuttle construct containing an acyclovir responsive component and a primate CD3ζ promoter. As initial prototypes, anti-ECD of EPCAM or ERBB2 scFv with CD8-α signal peptide, stalk and transmembrane domain were utilized. Solid tumor microenvironment-restricted CAR products were generated using methods as described in US Patent No. 8,709,755 and PCT Publication No. WO / 2016 / 033331A1 or by direct selection from human phage libraries under licensed and non-licensed conditions. Briefly, human V from Creative Biolabs (Shirley, New York) was panned under the following tumor license conditions H ×V L Libraries: 100 μg / ml hyaluronic acid, 100 kDa fragments (Lifecore Biomedical, Chasca, MN), 20 mg / ml recombinant HAS (Cyagen, Santa Clara, CA), 200 ng / ml recombinant human V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com