Application of AD16 in preparation of medicine for relieving chronic inflammatory pain
A technology of chronic inflammation and chronic inflammation, which is applied in the application field of preparing drugs for reducing chronic inflammatory pain, can solve the problems of long treatment course, variable clinical manifestations, and large side effects, and achieve mechanical pain relief, thermal pain relief, The effect of improving the degree of tissue swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Experimental grouping and processing:
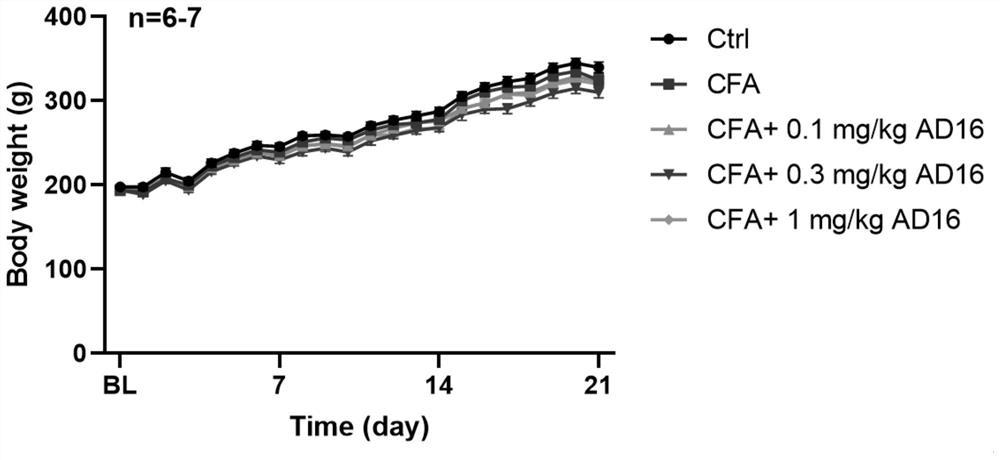
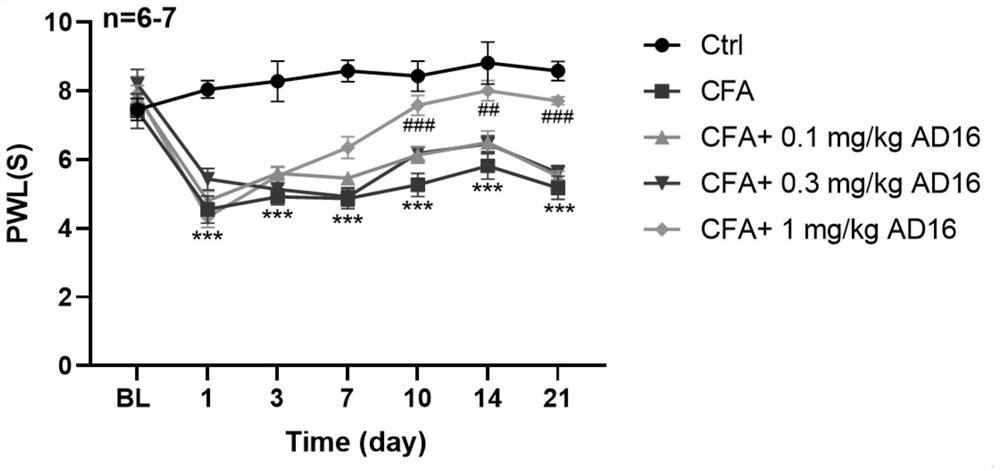
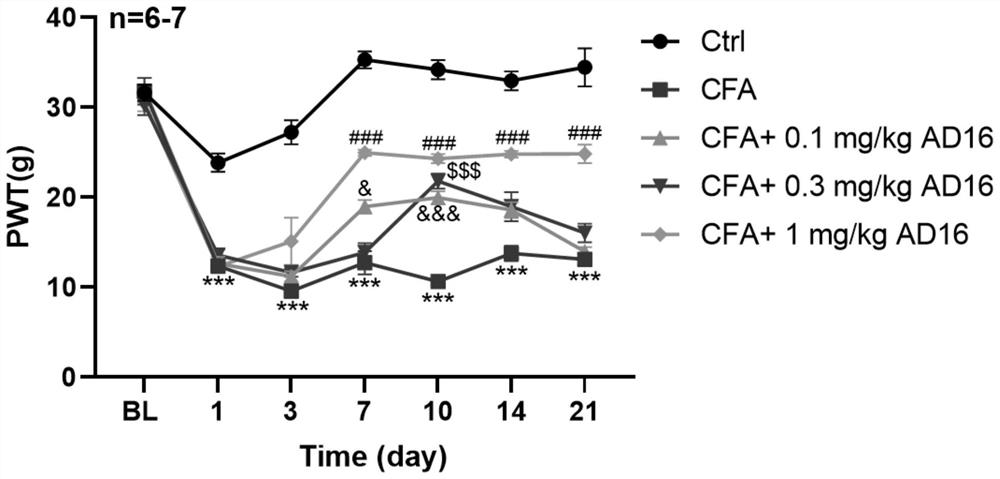
[0026] 31 SD rats (150-170g) were randomly divided into 5 groups, 6-7 rats in each group, respectively control group (Ctrl), model group (CFA group), 0.1mg / kg AD16 treatment group, 0.3mg / kg AD16 treatment group, kg AD16 treatment group, 1mg / kg AD16 treatment group. The model group and the AD16 treatment group were subcutaneously injected with 100 μL of Complete Freund's adjuvant (CFA) once in the left hind foot to prepare animal models of chronic inflammatory pain, and the control group was injected with the same volume of normal saline subcutaneously in the left hind foot. On the second day after modeling, the AD16 treatment group was given AD16 solutions with concentrations of 0.1, 0.3, and 1 mg / mL (DMSO as the solvent), and the volume of administration was 1 mL / kg. The final doses of AD16 were: 0.1, 0.3, and 1 mg / kg, respectively. The control group and the model group were intraperitoneally injected with the same volume of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com