Anti-cd117 antibodies and uses thereof
An antibody, T114 technology, applied in the direction of antibodies, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problems of difficult implantation of hematopoietic stem cell transplants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0632] Preparation of antibody-drug conjugates
[0633] In the ADC of Formula I disclosed herein, the anti-CD117 antibody or antigen-binding fragment thereof is linked to one or more cytotoxic drug moieties (D) (e.g., about 1 to about 20 drug moieties) via a linker L and a chemical moiety Z disclosed herein. / antibody) conjugation. The ADCs of the present disclosure can be prepared by several routes using organic chemistry reactions, conditions and reagents known to those skilled in the art, including: (1) Reactive substituents of antibodies or antigen-binding fragments thereof are reacted with bivalent linker reagents to Form Ab-Z-L as described above, then react with the drug moiety D; or (2) the reactive substituent of the drug moiety is reacted with a divalent linker reagent to form D-L-Z, and then react with the antibody or its Reactive substituents of the antigen-binding fragment react to form an ADC of formula D-L-Z-Ab, such as Am-Z-L-Ab. Additional methods of making ...
Embodiment 1
[0661] Example 1. Identification of Anti-CD117 Antibody 85 (Ab85)
[0662] Anti-CD117 antibody 85 (ie, Ab85) was identified based on the generation of a derivative of the human CK6 antibody (ie, Ab1 ) as described in PCT / US2018 / 057180 (the disclosure of which is incorporated herein by reference in its entirety). Antibody 85 is an antagonistic antibody, which can be determined by assaying the ability of Ab85 to block CD117-mediated activation, as known to those skilled in the art, comprising the amino acid sequence provided below. Briefly, cells that depend on the CD117 ligand SCF1 for proliferation (primary CD34+ cells or Mo7e cells) can be incubated in the presence of CD117-binding antibodies to assess their ability to block the interaction between CD117 and SCF1, This results in a measurable reduction in cell proliferation. Alternatively, simple binding experiments can be performed to measure antagonism of CD117 and SCF1 binding events.
[0663] Antibody HC-85 / LC-85 (Ab85)...
Embodiment 2
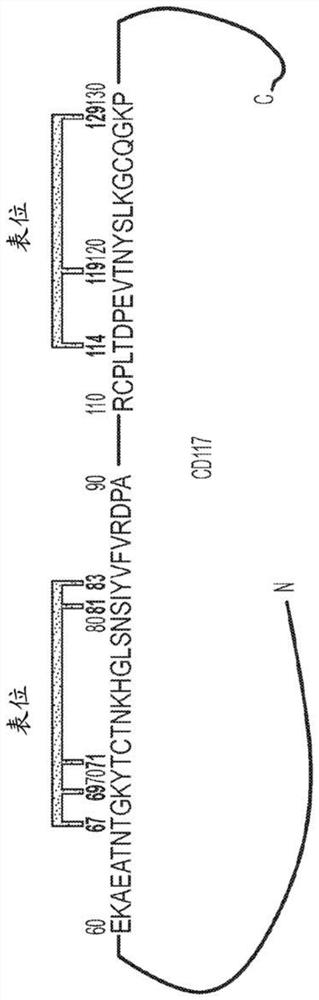
[0671] Example 2. Epitope analysis of Ab85
[0672] Chemical cross-linking experiment
[0673] Cross-linking experiments allow direct analysis of non-covalent interactions by high-quality MALDI mass spectrometry. By mixing protein samples containing non-covalent interactions with a specially developed cross-linking mixture (Bich, C et al. Anal. Chem., 2010, 82(1), pp 172–179), it is possible to specifically detection of non-covalent complexes. The resulting covalent binding allows the interacting species to survive the sample preparation process and MALDI ionization. A special high-mass detection system allows the characterization of interactions in the high-mass range.
[0674] Ab85 samples were diluted in distilled water to a concentration of 2.7 mg / mL. 1 μL of diluted Ab85 was then mixed with 1 μL of matrix consisting of recrystallized sinapinic acid matrix (10 mg / mL) in acetonitrile / water (1:1, v / v), TFA (0.1% (K200MALDI Kit). The mixture was crosslinked using CovalX'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com