Chimeric autoantibody receptor (CAAR) that binds autoantibodies targeting the central nervous system in neurological autoimmune disease
A technology for autoimmune diseases and autoantibodies, applied in nervous system diseases, allergic diseases, antibody medical components, etc., can solve problems such as lack of experimental support, reduce the risk of infection or sepsis, and reduce toxic immune side effects , the effect of reducing the risk of clinical recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0321] Example 1: Generation of NMDAR-CAAR constructs and corresponding CAAR-T cells
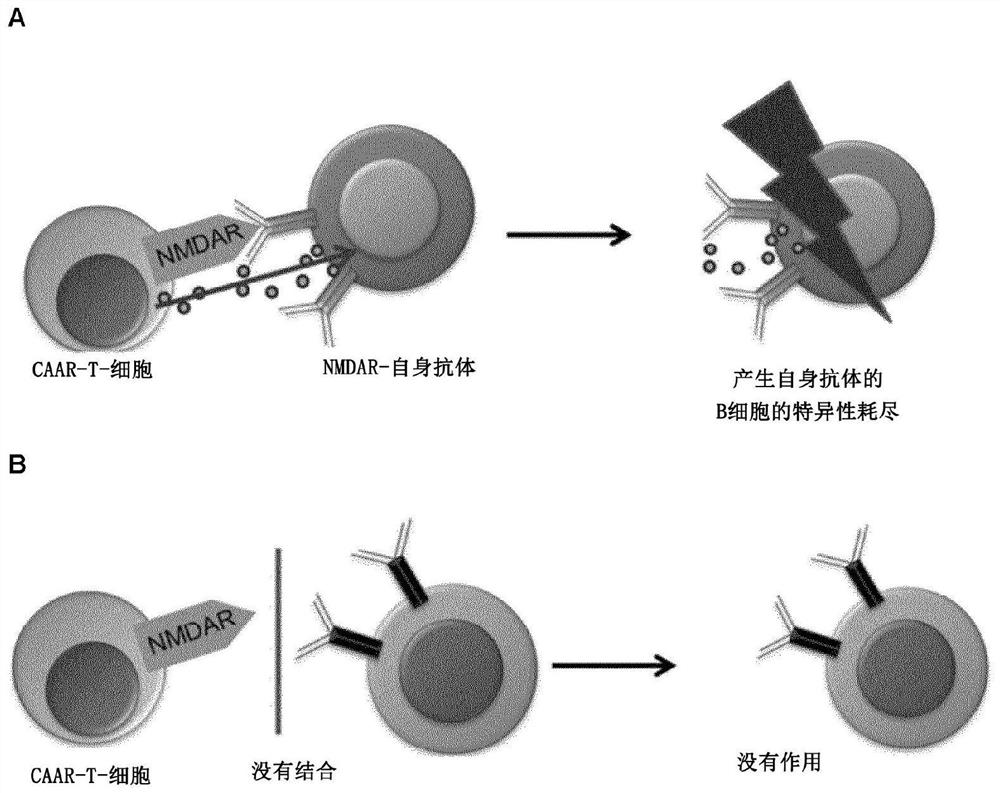
[0322] A schematic outline of the method of the present invention is as figure 1 shown.
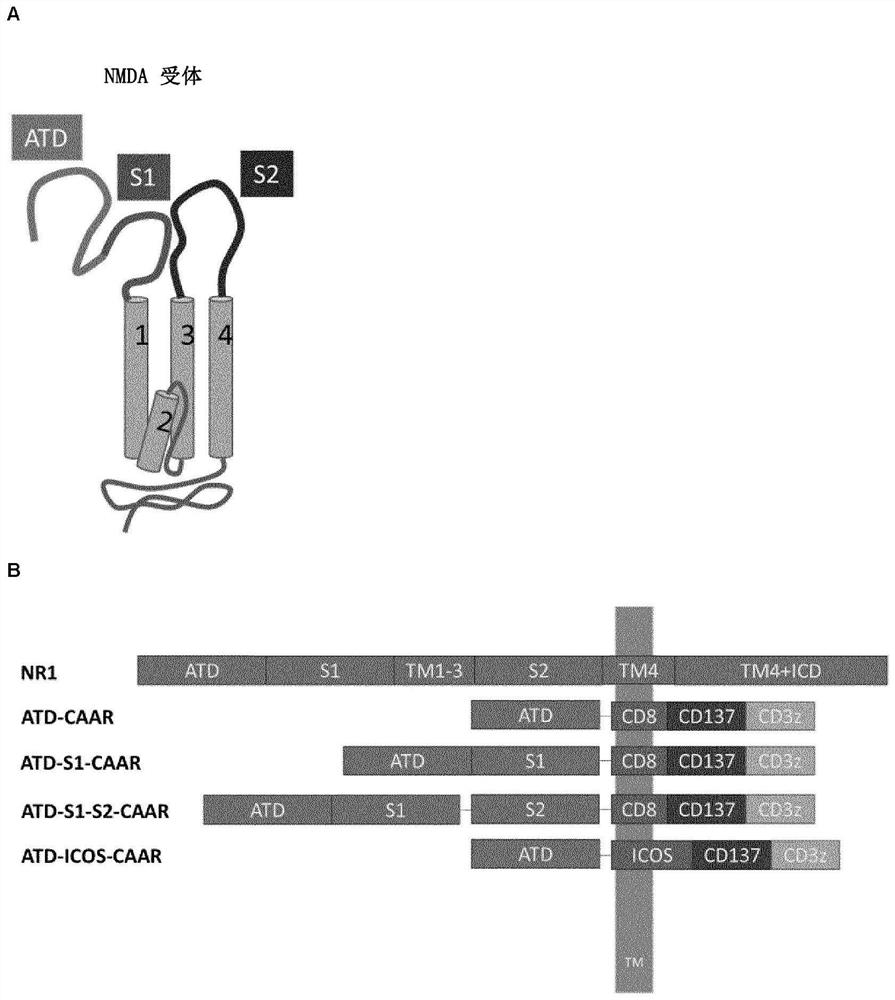
[0323] In order to demonstrate a practical non-limiting embodiment of the invention, the inventors created several CAAR-T constructs ( figure 2 ). These constructs are based on the backbone of the CAR vector ( figure 2 B). The domain of the NMDA receptor has been localized in the CAR vector, replacing the conventional antibody fragment usually contained in the CAR vector.
[0324] To this end, various combinations of immune-relevant extracellular NMDA receptor domains were cloned into CAR constructs ( figure 2 A). Such as figure 2 Shown in A, the amino-terminal domain (ATD) and domains S1 and S2 of the NR1 subunit of the NMDA receptor were used in place of the typical antigen-binding antibody fragment of the CAR construct, resulting in a chimeric autoantibody receptor (CAAR) Constructs in wh...
Embodiment 2
[0328] Example 2: Activation of CAAR-T cells by clustered anti-NMDAR NR1 antibodies
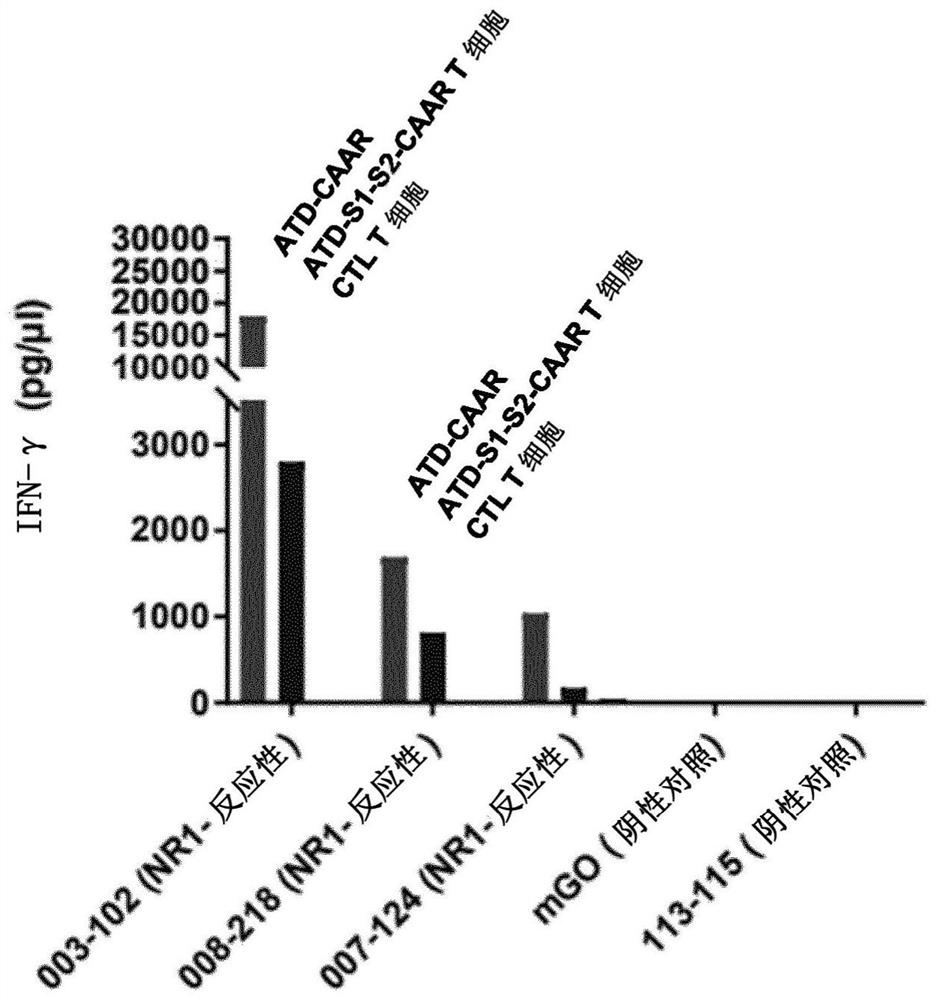
[0329] For this, ELISA plates were coated with human NMDAR antibodies and then incubated with CAAR-T cells or control T cells. Activation of CAAR-T cells results in the release of interferon-γ, which is measured in the supernatant.
[0330] image 3 It was shown that strong release of interferon-γ was clearly visible only when NMDAR antibodies (003-102, 008-218) bound to CAAR-T cells (left column in the figure). No significant amounts of interferon-γ were detected in samples on ELISA plates coated with control antibodies (mGo, 113-115), or in samples incubated with NMDAR antibodies and control T cells (right column in the figure).
Embodiment 3
[0331] Example 3: Activation of CAAR-T cells by NMDAR NR1 antibody presented on the surface of HEK or K562 cells
[0332] To this end, the inventors employed a previously established human cell model producing NMDA receptor antibodies. In this model, HEK293 cells express a human monoclonal NMDA receptor antibody localized to their cell membrane. The sequence of a human NMDA receptor antibody was previously determined (Kreye et al., 2016).
[0333] Figure 4showed that, similar to the assay described in Example 2, strong activation of CAAR T cells was observed only in samples subjected to co-culture with target cells for 48h (upper panel) or 24h (lower panel) (left column in the figure) is evident, corresponding to a large release of interferon-γ, but could not be observed in samples co-cultured with HEK wild-type cells or co-cultured with control T cells (right column in the figure).
[0334] Figure 5 showed that co-culture of CAAR T cells at a ratio of 1:1 with K562 cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com