Injectable polymer nanoparticle compositions of antithrombotic agents and methods thereof
A nanoparticle and composition technology, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
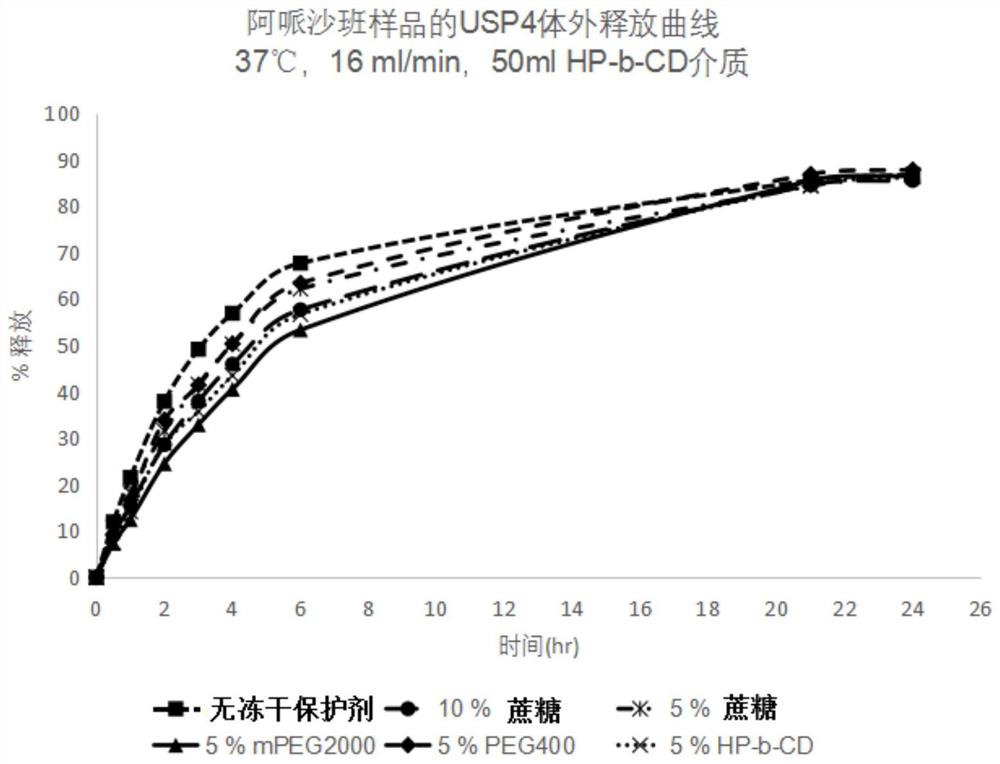
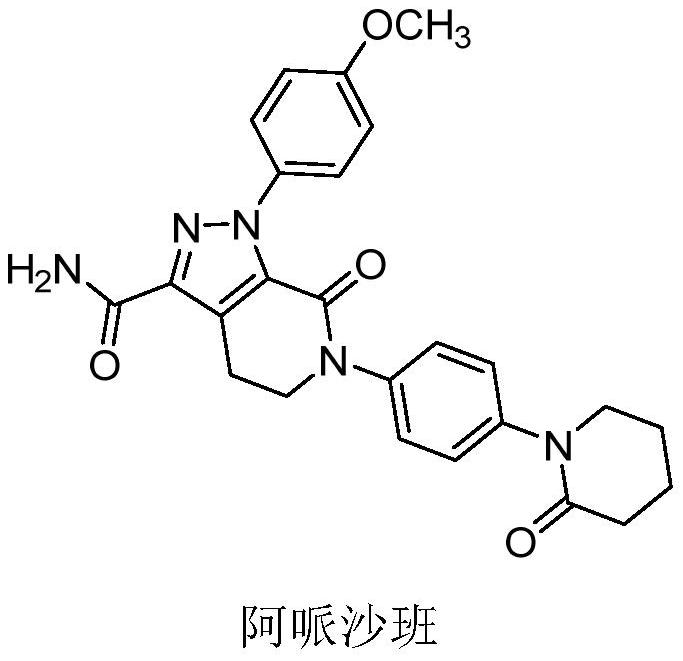
[0087] Example 1. General preparation of mPEG-PLA polymer micelles containing apixaban The method used for the preparation of polymer micelles containing apixaban can refer to Int. 206 and J. Control. Release, 2001, 72, 191-202.
[0088] Briefly, apixaban (7.7 mg) and mPEG-PLA (770 mg, molecular weight = 3860-4200 Daltons) were dissolved in 5 mL of methanol / dichloromethane (3 / 7 v / v) mixed solvent. Then the organic solvent was removed under reduced pressure at 60°C to obtain a transparent gel matrix, which was dissolved by adding 25mM citrate buffer (pH 6.1) at 60°C to form a transparent apixaban-encapsulated micellar solution. The solution was sterilized by filtration through a 0.22 μm membrane and then lyophilized using a freeze dryer system.
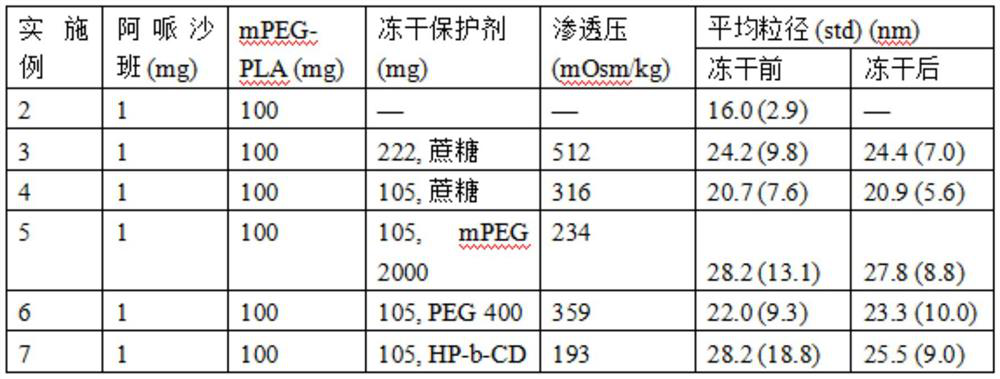
[0089] An average micelle size of 19.8 nm was observed at a concentration of apixaban of 0.5 mg / mL. One or more lyoprotectants are dissolved in the apixaban solution. The resulting apixaban-lyoprotectant solution was then lyophilize...
Embodiment 2
[0090] Example 2. Apixaban-containing polymer micelle composition was prepared by the method described in Example 1 using the following ingredients:
[0091] mPEG-PLA (molecular weight = 3860-4200 Daltons), 100 mg.
[0092] Apixaban, 1mg
[0093] water, 2mL.
Embodiment 3
[0094] Example 3. Apixaban-containing polymer micelle composition was prepared by the method described in Example 1 using the following ingredients:
[0095] mPEG-PLA (molecular weight = 3860-4200 Daltons), 100mg
[0096] Apixaban, 1mg
[0097] 25mM citrate buffer (pH 6.1), 2mL.
[0098] Sucrose: 222mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com