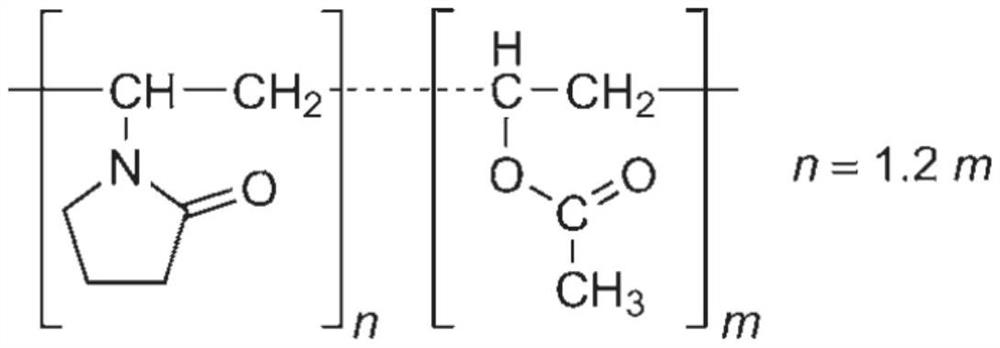
Vonoprazan fumarate pharmaceutical composition and preparation method thereof
A composition, fumaric acid technology, applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., can solve the problems of easy melting and affect the stability of the drug vonolax fumarate, so as to control growth and ensure stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Prescription ingredients and ratio:
[0069] table 3
[0070]
[0071]
[0072] Preparation method: same as Comparative Example 1.
[0073] Investigation of influencing factors: same as Comparative Example 1.
Embodiment 2
[0074] Embodiment 2 Influencing Factors Investigation Result Determination
[0075] Related Substance Detection Method
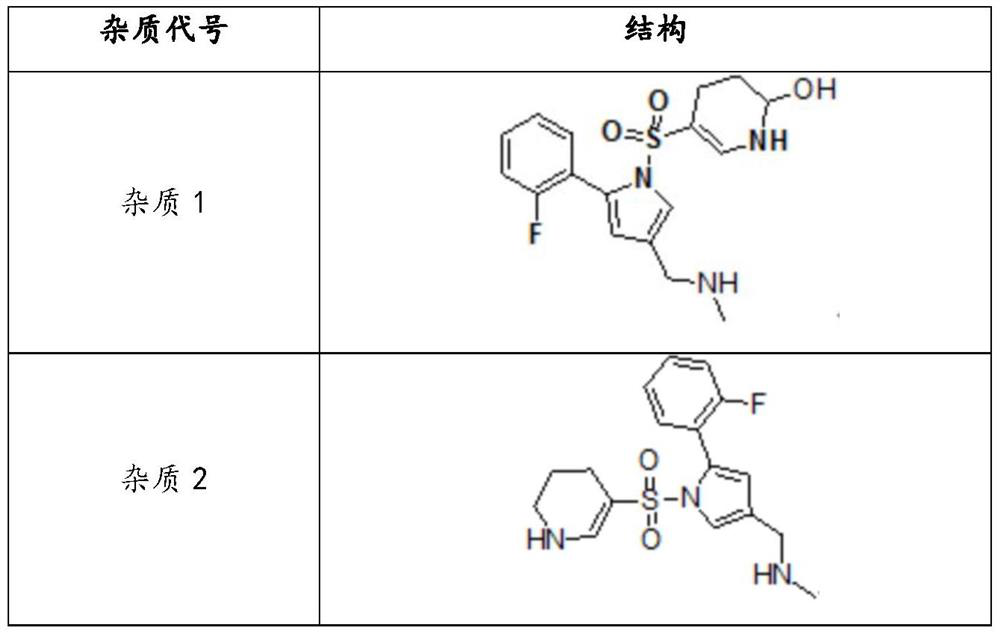
[0076] The prepared samples were tested for changes in known impurities and unknown impurities in the raw vonolamin fumarate tablets of Comparative Example 1 and Example 1 before and after setting out. By HPLC method, water: acetonitrile mixture (3:1) was used as solvent to extract related impurities for determination. HPLC test condition is as follows:
[0077] Detector: UV absorption spectrophotometer (measurement wavelength: 230nm)
[0078] Chromatographic column: Octadecylsilane bonded silica gel column [MGIIC18 (4.6×100mm, 3μm)]
[0079] Mobile phase A: 0.025mol / L phosphoric acid-acetonitrile-methanol mixture (14:1:5)
[0080] Mobile phase B: 0.025mol / L phosphoric acid-acetonitrile mixture (3:7)
[0081] Mobile phase: Gradient elution by changing the mixing ratio of mobile phase A and mobile phase B as follows:
[0082] Table 4
[0083]
[008...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap