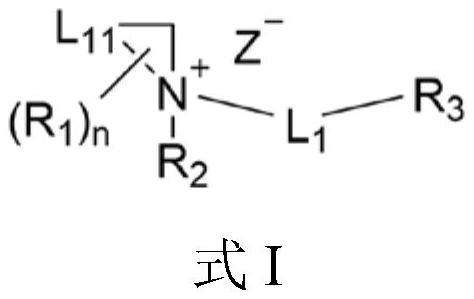
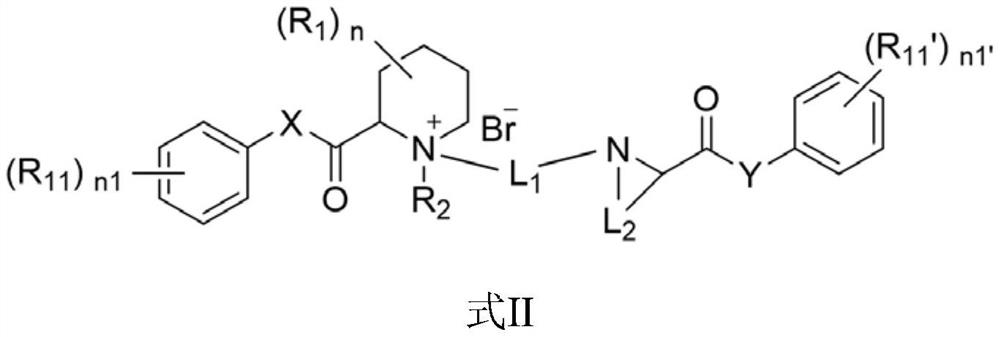
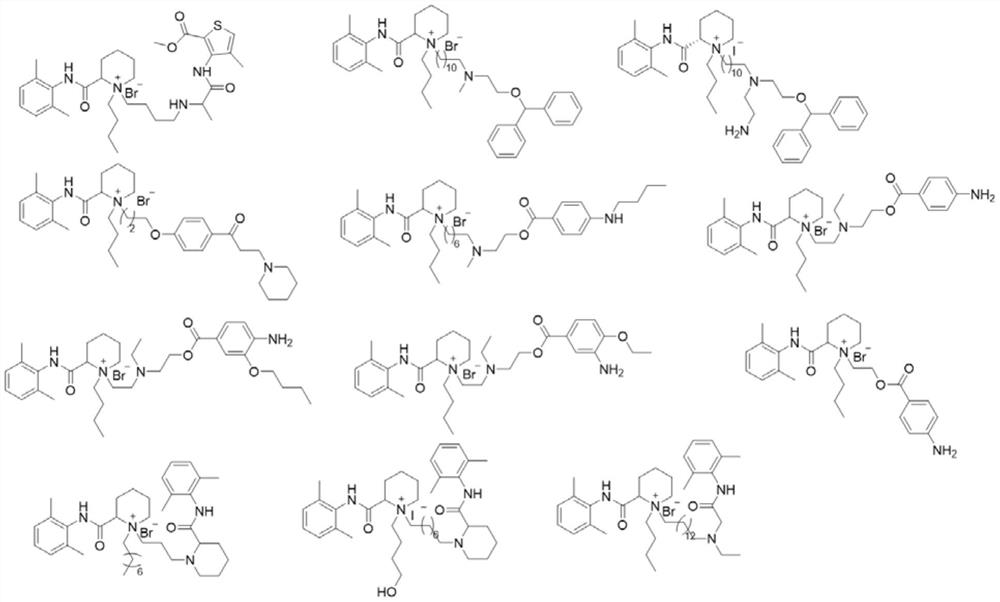
Quaternary ammonium salt compound for anesthesia as well as preparation method and application thereof
A compound and solvate technology, applied in the field of medicinal chemistry, can solve the problems of increasing medical costs and the incidence of infection, the inability to recover motor function after surgery, and limiting the wide application of local anesthetics, etc., to achieve long-term local anesthesia , Long-acting selective local anesthesia, long-term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0283] Example 1, Preparation of Compounds of the Invention
[0284]
[0285] Compound 1a (10.0 g, 40.59 mmol) was dissolved in 15 mL of 1,3-dibromoles, heated to 70 ° C for 48 h, TLC monitoring (DCM: MeOH = 10: 1, V / V, R) f = 0.3). The appropriate amount of petroleum ether was added, and the viscous syrup-like substance was formed, poured out of the upper tank, and 13.5 g of the remaining crude product was dissolved with 25 ml of methanol, and the silica gel was simatted after drying. Eluent: DCM: MeOH = 10: 1, V / V, collect eluting eluting eluting eluting eluting eluting eluting elut elut elut Ethyl acetate and dichloromethane were recrystallized to prepare a 8.8 g of a white solid powder (intermediate 1b) with a yield of 58.9% for the next reaction.
[0286] The above-mentioned intermediate 1b (1.00 g, 2.17 mmol), Sn- (2,6-dimethylphenyl) -2-piperidine (0.505 g, 2.17 mmol) was dissolved in 10 ml of ethanol, DIPEA (0.99 g, 0.78 mL, 4.74 mmol) was added, warmed to 80 ° C, he...
Example Embodiment
[0287] Example 2, Preparation of Compounds of the Invention
[0288]
[0289] Compound 2a (10.0 g, 40.59 mmol) was dissolved in 15 mL of 1,5-dibromoprice, heated to 75 ° C for 36 h, TLC monitoring (DCM: MeOH = 10: 1, V / V, R) f = 0.3). The appropriate amount of petroleum ether was added, and the viscous syrup-like substance was formed, poured out of the upper tank, and the remaining crude product was dissolved, and after dissolving with silica gel, the silica gel column chromatography was purified. Eluent: DCM: MeOH = 10: 1, V / V, collect eluting eluting eluting eluting eluting eluting eluting eluting eluting elut Ethyl acetate and dichloromethane were recrystallized to prepare 10.1 g of a white solid powder (intermediate 2b), the yield was 62.8% for the next reaction.
[0290] The above-mentioned intermediate 2b (1.00 g, 2.52 mmol), Sn- (2,6-dimethylphenyl) -2-piperidine (0.585 g, 2.28 mmol) was dissolved in 10 ml of ethanol, DIPEA (0.99 g, 0.78 mL, 4.74 mmol) was added, warm...
Example Embodiment
[0291] Example 3, Preparation of Compounds of the Invention
[0292]
[0293]Compound 3A (10.0 g, 34.67 mmol) was dissolved in 15 mL of 1,5-dibromoprice, heated to 75 ° C for 48 h, TLC monitoring (DCM: MeOH = 10: 1, V / V, R) f = 0.3). The appropriate amount of petroleum ether was added, and the viscous syrup-like substance was added, poured out of the upper layer, 14 g of the remaining crude product, mixed with silica gel after dissolving using 25 ml of methanol, and the silica gel column chromatography was purified. Eluent: DCM: MeOH = 10: 1, V / V, collect eluting eluting eluting eluting eluting eluting eluting elut elut elut Ethyl acetate and dichloromethane were recrystallized to prepare 8.6 g of a white solid powder (intermediate 3b), yield 56.7% for the next reaction.
[0294] The above-mentioned intermediate 3b (1.00 g, 2.28 mmol), Sn- (2,6-dimethylphenyl) -2-piperidine (0.530 g, 2.28 mmol) was dissolved in 10 ml of ethanol, DIPEA (0.99 g, 0.78 mL, 4.74 mmol) was added, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap