2, 5-thiophene-diformaldehyde-2-amino-4-methylphenol Schiff base and preparation method and application thereof
A technology of methyl phenol and diformaldehyde, applied in 2 fields, can solve the problem of less synthesis of bis-Schiff bases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
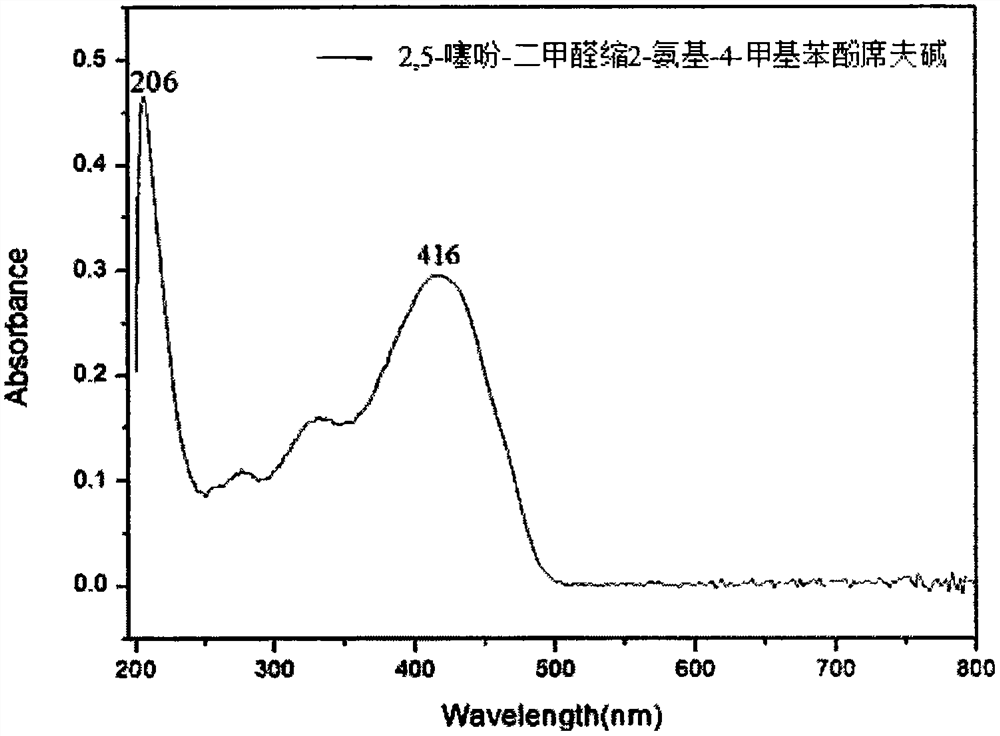
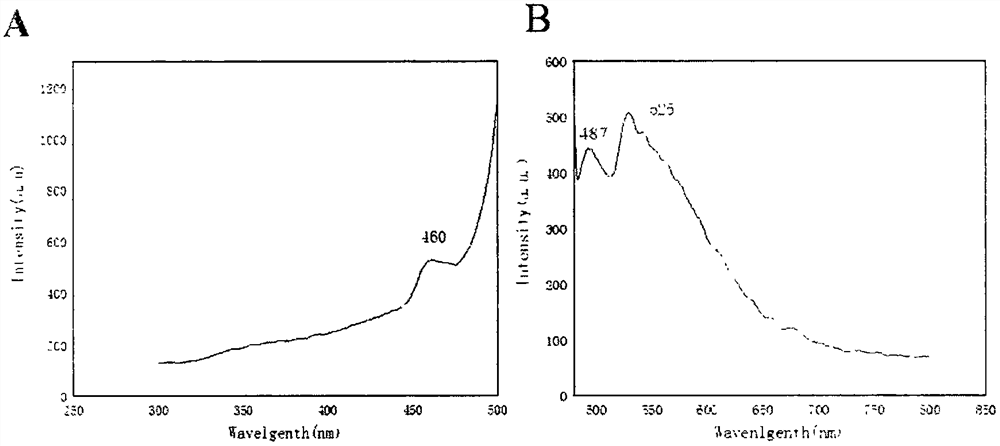
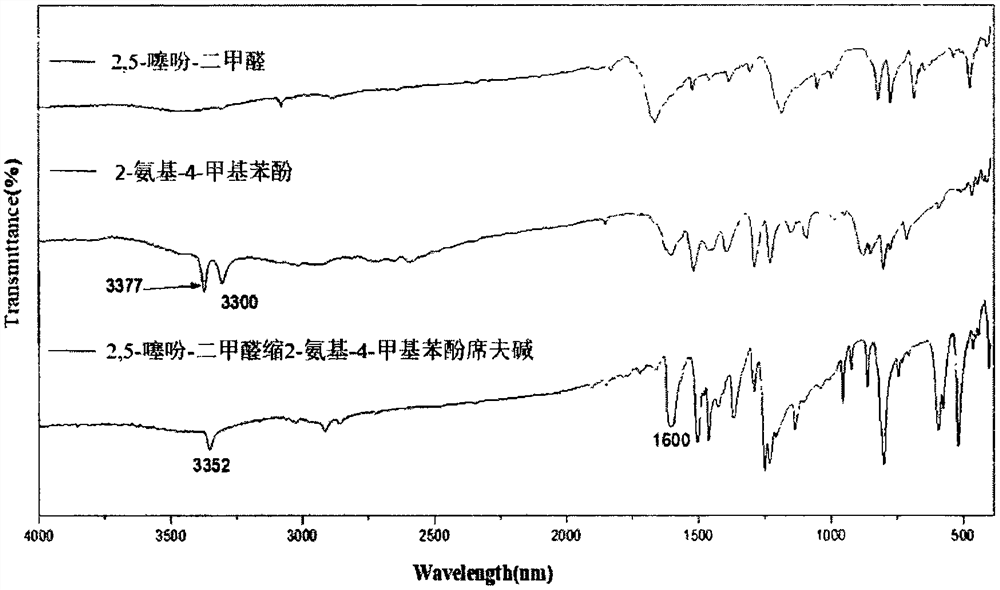
[0025] Example 1 The preparation method of 2,5-thiophene-dimethylaldehyde acetal 2-amino-4-methylphenol Schiff base compound:
[0026] Weigh 0.140g of 2,5-thiophene-diformaldehyde and dissolve it in 20.0mL of absolute ethanol to obtain a 2,5-thiophene-diformaldehyde solution, weigh 0.246g of 2-amino-4-methylphenol and dissolve it in 20.0mL Prepare 2-amino-4-methylphenol solution with absolute ethanol, add 2-amino-4-methylphenol solution dropwise to 2,5-thiophene-diformaldehyde solution and react at 50°C for 2 hours to form crystals , filtered with suction, washed with ethanol, and dried naturally to obtain crystals, then washed with 50.0 mL of absolute ethanol, and dried at 50° C. for 1 h to obtain orange-yellow crystals.
[0027] The crystal is 2,5-thiophene-dimethylaldehyde acetal 2-amino-4-methylphenol Schiff base, which is composed of one molecule of 2,5-thiophene-dimethylaldehyde and two molecules of 2-amino-4- Schiff base formed by condensation of methylphenol.
[0028...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com