Pharmaceutical composition for treating hepatocellular carcinoma and application thereof
A hepatocellular carcinoma and composition technology, applied in the field of medicine, can solve problems such as high concentration of drugs in the body, difficulty in tolerance of patients, lack of targeting of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of liposomal nanoparticle (LNP-DP1)
[0040] Parameters such as the raw material components and dosage of the liposomal nanoparticles (LNP-DP1) used in this example are shown in Table 1.
[0041] Table 1 Liposome nanoparticle (LNP-DP1) preparation raw material
[0042]
[0043]
[0044] The preparation method of liposomal nanoparticles (LNP-DP1) is as follows:
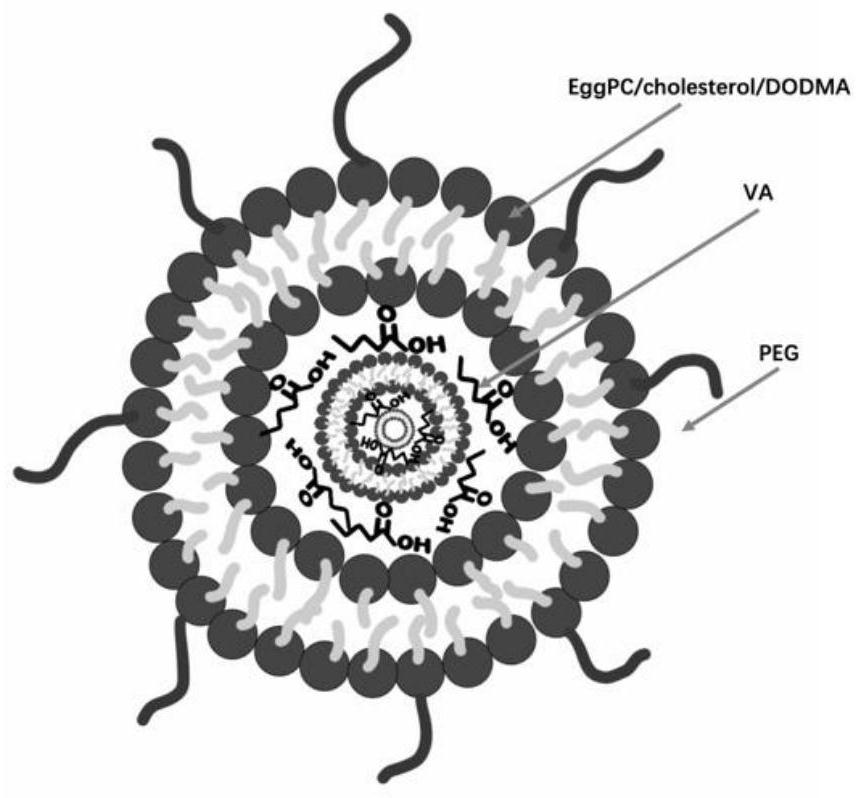
[0045] Day 1: Preheat alcohol with a concentration of 100% in a water bath to 60°C, weigh all the liquid components in Table 1 according to the molar ratio, add them to the preheated alcohol as required, and fully dissolve them. Dissolve the four liquid components in proportion into an alcohol solution (DODMA / EggPC / Chol / PEG-liqid=45:15:35:5) (dissolve in a water bath at 60°C), slowly drop the mixed solution into it with a syringe and stir quickly In the HEPES solution (20mM, pH7.4), until the solution is a new mixed liquid containing 35% alcohol concentration. MWCO 10,000 Dal...
Embodiment 2
[0054] Example 2: Preparation of liposomal nanoparticles coated with valerenic acid
[0055] Add an equal volume of LNP-DP1 and valerenic acid (mixed at a volume ratio of 1:1) into a centrifuge tube, centrifuge at 2100g for 20 min, centrifuge the nanoparticle valerenic acid complex and concentrate it to 200 μl, centrifuge and let it stand for 20 min before use . Dilute and add medicine according to the required concentration, and prepare freshly before each use. Liposome nanoparticles coated with valerenic acid (LNP-DP1-valerenic acid, referred to as LV) complexes such as figure 1 shown.
Embodiment 3
[0056] Embodiment 3: pharmacological research
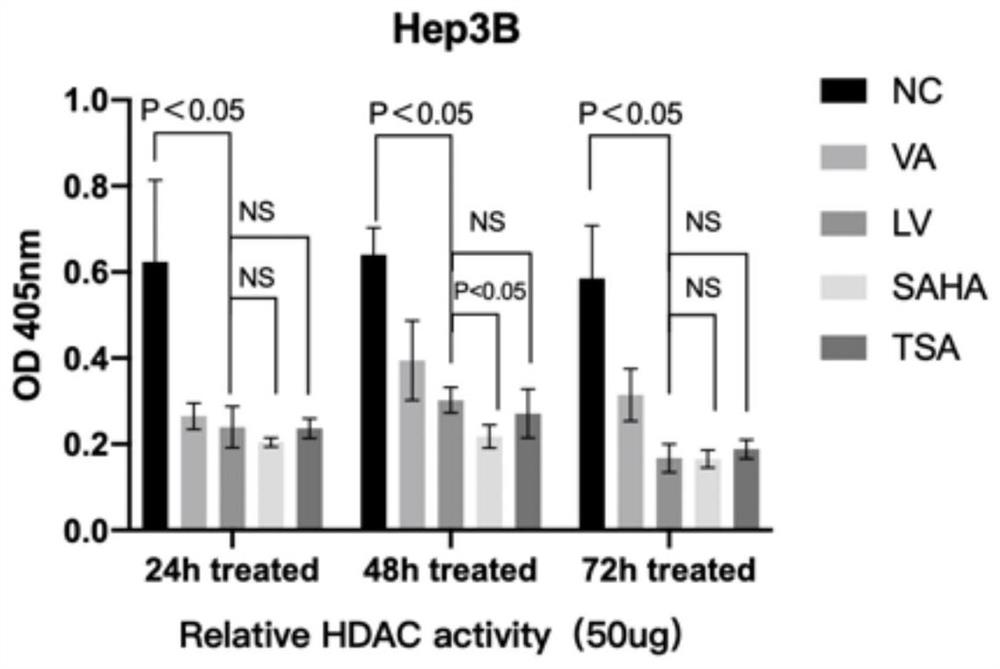
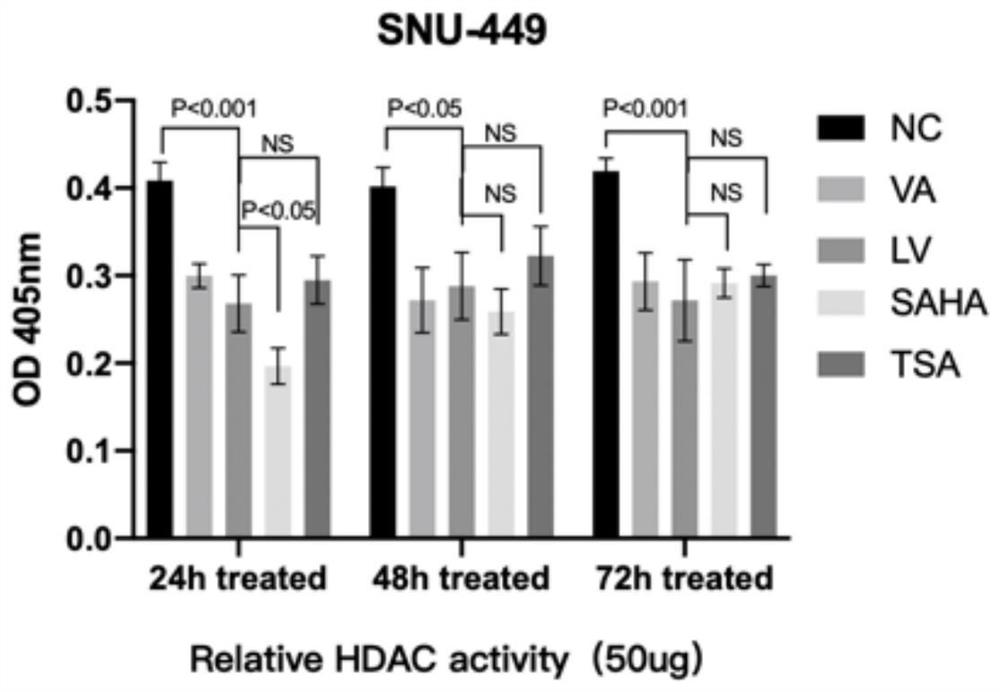
[0057] Effects of Valerenic Acid (VA) on HDAC Activity in Liver Cancer Cells
[0058] The HDAC Activity Colorimetric kit (BioVision, USA) was used to detect the effect of valerenic acid (VA) and liposome nanoparticles (LV) coated with valerenic acid on the HDAC activity of M test cells. Dilute the sample to be tested (50-200 μg cell lysate) with double distilled water to 85 μl (final volume) per well. Add 10 μl of 10X HDAC Assay Buffer to each well. 5 [mu]l of HDAC colorimetric substrate was added to each well and the solution was allowed to mix thoroughly. Place the plate to be tested in the incubation phase at 37°C for 1 hour (the incubation time can be extended as appropriate). Add 10 μl Lysine Developer to each well and mix well to stop the reaction. Continue to place the plate to be tested in a 37°C incubator for 30 minutes. Using an Epoch microplate spectrophotometer (Bio-Tek, USA), the OD value of the sample was measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com